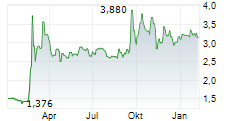
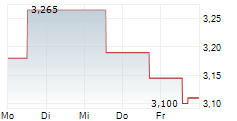
Oryzon Genomics has announced that it has received FDA feedback on its plans for the Phase III programme in borderline personality disorder (BPD) for its lead central nervous system (CNS) drug candidate, vafidemstat. Following the submission of the proposed protocol in June 2025, Oryzon now has written feedback regarding various components of the programme, including trial endpoints as well as non-clinical considerations. Management has communicated that this dialogue with the regulators has been constructive and will enable the company to re-submit a revised Phase III protocol in due course. We highlight that such interactions with the FDA are common in drug development, and perhaps unsurprising in this case where there is no regulatory precedent for BPD. Should the FDA clear Oryzon's revised protocol, it should strengthen the company's chances of bringing an effective new treatment option for patients with BPD, an indication that currently has no approved drugs.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research