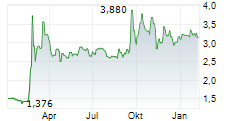
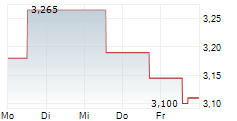
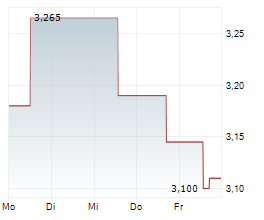
Oryzon Genomics has reported its Q325 results, a period characterised by tangible progress across its ongoing clinical programmes. Anticipation builds for vafidemstat in borderline personality disorder (BPD) following the receipt of feedback from the FDA for the proposed Phase III PORTICO-2 programme; interactions with the regulators have been constructive and Oryzon plans to resubmit a revised protocol. Oryzon's oncology-haematology candidate, iadademstat, reported positive clinical data in acute myeloid leukaemia (AML) and further details are due to be presented at ASH 2025. We note that management aims to build the data package for iadademstat to support its efforts in seeking a partner, as part of a renewed strategy to become a central nervous system (CNS) specialist. Following the Q3 results, our valuation shifts to €909.3m or €11.4 per share (from €887.2m or €11.3 per share previously).Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research