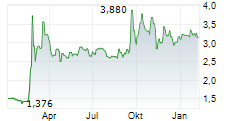
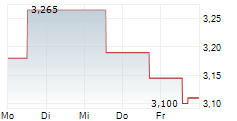
Oryzon has announced encouraging updates for iadademstat. In acute myeloid leukaemia (AML), positive data was reported for two programmes, including the lead oncology programme, FRIDA. Updated interim data from FRIDA (iadademstat in combination with gilteritinib in relapsed/refractory AML) showed an overall response rate (ORR) of 67%, suggesting improved outcomes compared to gilteritinib alone. In a separate Phase I study exploring the synergy between iadademstat, venetoclax and azacitidine, in newly diagnosed AML, the preliminary interim data (n=8) showed a 100% ORR. We view this as a positive indicator of the novel combination, but we acknowledge that it is from a relatively small population. Beyond malignant haematological indications, Oryzon has also enrolled the first patient in its sickle cell disease (SCD) trial. If successful, we believe this could bolster the value proposition for iadademstat, with applications beyond oncology.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research