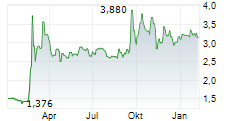
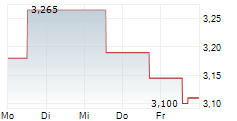
Q125 saw Oryzon make significant headway towards Phase III for vafidemstat in BPD and clinical advancement for iadademstat across multiple early-stage studies. Operating performance was unsurprising, with opex rising slightly (+5.6% y-o-y to €3.4m) as the company continues to prepare for submitting the vafidemstat Phase III study protocol to the FDA (expected in Q225). The liquidity position has improved materially so far in Q2, supported by a €30m equity raise and another €13.3m in grant income. We estimate that pro forma gross funds (c €47m, including end-Q125 cash of €3.8m) will provide runway through 2027, excluding the BPD trials, which we model will be funded by a licensing partner. We adjust our launch timelines for the iadademstat programmes based on current visibility, with our valuation shifting modestly to €862.4m (€885.1m previously). We adjust our per-share valuation to €11.0 from €13.5, reflecting the increased number of shares outstanding following the equity raise.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research