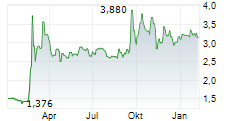
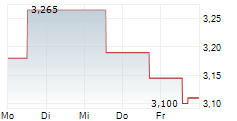
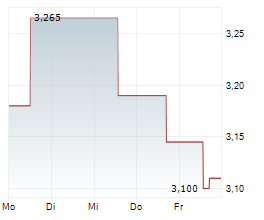
Oryzon Genomics has reported its Q225 results, summarising an active period. Its programme focused on vafidemstat in borderline personality disorder (BPD) remains a strategic priority, with the Phase III protocol submitted to the FDA in June. Clearance is anticipated in Q325, most likely in September, and could represent the most significant upcoming inflection point for the company. Trials relating to Orzyon's lead oncology candidate, iadademstat, continue to progress, with the next FRIDA update in acute myeloid leukaemia (AML) expected in December 2025. Oryzon has also expanded iadademstat's potential application to new non-malignant haematological indications; a clinical trial application (CTA) for a Phase Ib study in sickle cell disease (SCD) has been submitted to the EMA. Following the Q225 results update, our valuation adjusts to €887.2m or €11.3 per share (from €862.4m or €11.0 per share previously).Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research