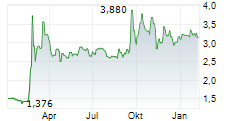
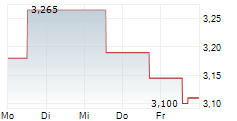
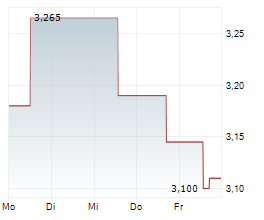
At Oryzon Genomics' key opinion leader (KOL) event, vafidemstat's path through Phase III in borderline personality disorder (BPD) was laid out. The significant unmet need was highlighted, as there are currently no FDA-approved drugs specifically indicated for BPD, despite a global prevalence of 1-2%, and clinicians are limited to prescribing medications (such as antipsychotics and/or mood stabilisers) off-label, often with limited durable effectiveness. Given vafidemstat's encouraging track record in the clinic targeting agitation and aggression (A/A) in BPD, alongside its novel epigenetic mechanism of action, the KOLs spoke about their willingness to use vafidemstat with their patients, especially since this symptom domain is one of the most common (70% of patients) and debilitating components of the condition, both for the patients and people around them. Oryzon submitted the protocol for the Phase III PORTICO-2 programme to the FDA in June 2025 and management anticipates receiving regulatory feedback within Q425, potentially representing an important upcoming catalyst.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research