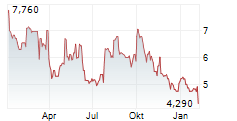
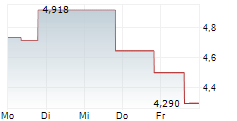
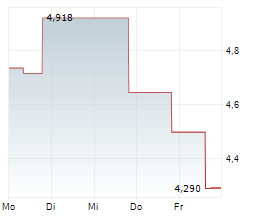
OSE Immunotherapeutics has reported positive top-line data from the investigator-sponsored Phase II trial assessing Tedopi in combination with FOLFIRI (a three-drug chemotherapy regimen made up of folinic acid, fluorouracil and irinotecan) in patients with pancreatic cancer (advanced or metastatic pancreatic ductal adenocarcinoma, PDAC). Importantly, the trial met the primary endpoint of one-year overall survival and demonstrated favourable safety outcomes, laying a robust foundation for further development efforts, in our view. Follow-up and translational analyses are ongoing, and management plans to communicate more detailed results at conferences in the coming months. We remind readers that the lead Tedopi programme is focused on non-small cell lung cancer (NSCLC); the registrational Phase III ARTEMIA trial is ongoing and due to conclude in 2027.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research