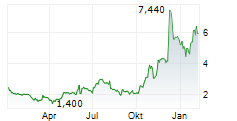
Immix's FY24 results summarize a period of focus on its lead CAR-T asset, NXC-201, which is being developed for amyloid light chain amyloidosis. Recent newsflow reflects heightened clinical activity for the candidate as it progresses through the US-based Phase Ib/II NEXICART-2 clinical trial. Notably, following completion of the six-patient safety run-in portion of the study, the pace of enrolment has picked up for participants to be treated at the higher of the two tested doses; we expect the next interim data update in mid-2025. NXC-201 was granted regenerative medicine advanced therapy (RMAT) designation by the FDA, which may streamline its clinical development and subsequent approval process. We expect period-end net cash of $17.7m to provide a runaway into Q425, past interim readouts for NXC-201. Our valuation is largely unchanged at $126.3m or $4.6/share.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research