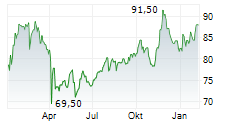
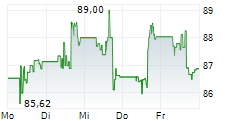
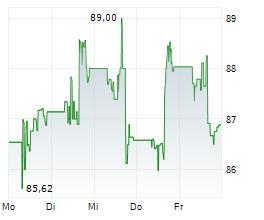
WASHINGTON (dpa-AFX) - Medtronic (MDT) revealed that its Expand URO Investigational Device Exemption (IDE) clinical study, the most extensive of its kind for robotic-assisted urologic surgery, successfully met its primary safety and effectiveness goals. The prospective, multi-center, single-arm IDE study included 137 patients who underwent urologic procedures using the Hugo robotic-assisted surgery (RAS) system.
The rates of grade 3 or higher complications (3.7% prostatectomy, 1.9% nephrectomy, and 17.9% cystectomy) were favorably below the performance goals, which were established based on systematic review of published literature across all three cohorts.
The study's 98.5% surgical success rate was well above the performance goal of 85% (p<0.0001), which was established based on a systematic review of published literature. There were two conversions: one was related to the device and one was related to patient anatomy.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News