| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
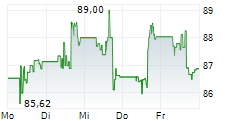
| 83,75 | 84,09 | 22:58 | |
| 83,77 | 84,02 | 22:00 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| MEDTRONIC Aktie jetzt für 0€ handeln | |||||
| Sa | Medtronic: The Only Medical Device Stock I'd Consider a Lifetime Hold | 15 | The Motley Fool | ||
| Sa | US IPO Week Ahead: Medtronic carve-out MiniMed to kick-off March calendar | 6 | Renaissance Capital | ||
| Sa | US IPO Week Ahead: Medtronic carve-out MiniMed to kick-off March activity | 5 | Renaissance Capital | ||
| Do | Medtronic reports positive findings for Altaviva tibial neuromod for urinary incontinence | 1 | MassDevice | ||
| Do | Is Medtronic Stock Underperforming the S&P 500? | 6 | Barchart.com | ||
| Mi | Medtronic launches MiniMed Go smart MDI system with Simplera sensor in Europe | 1 | MassDevice | ||
| Mi | Medtronic Launches MiniMed Go Smart MDI System In Europe | 276 | AFX News | WASHINGTON (dpa-AFX) - Medtronic (MDT) announced the commercial launch in Europe of the MiniMed Go Smart MDI system with the Simplera sensor. The launch will be rolled out gradually across Europe... ► Artikel lesen | |
| Mi | [GPTW] Medtronic empowers people for future of health care | 3 | Korea Times | ||
| Di | Medtronic plc - 10-Q, Quarterly Report | 3 | SEC Filings | ||
| Di | Medtronic MiniMed Diabetes unit prices IPO at up to $784M | 5 | MassDevice | ||
| Di | Medtronic diabetes business carve-out MiniMed sets terms for $742 million IPO | 3 | Renaissance Capital | ||
| Di | Medtronic: Improving Sustainability in Packaging & Products | 585 | ACCESS Newswire | Medtronic delivers business value through product and packaging improvements that conserve natural resources, increase recyclability, and help minimize emissions and waste. NORTHAMPTON, MA / ACCESS... ► Artikel lesen | |
| Di | Medtronic's diabetes unit MiniMed seeks up to $784M in U.S. IPO | 6 | Seeking Alpha | ||
| 23.02. | Medtronic wins CE mark for next-gen GI Genius module, ColonPRO software | 3 | MassDevice | ||
| 21.02. | The Only Healthcare Stock I Would Buy and Never Sell Is Medtronic | 12 | The Motley Fool | ||
| 20.02. | Medtronic Diabetes to lay off 81 employees ahead of MiniMed separation | 12 | MassDevice | ||
| 20.02. | Pharoah Neuro raises $20M, names former Medtronic exec as CEO | 2 | MassDevice | ||
| 19.02. | Medtronic: Kostenerstattung für Bluthochdrucktherapie in Japan genehmigt | 3 | Investing.com Deutsch | ||
| 19.02. | Medtronic renal denervation has a reimbursement win in Japan | 3 | MassDevice | ||
| 18.02. | Truist senkt Kursziel für Medtronic nach Ausblick | 36 | Investing.com Deutsch |
1 2 3 4 5 Weiter >>
360 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| FRESENIUS | 50,78 | -1,05 % | Fresenius: Weiteres Wachstum in Sicht - Aktie bärenstark | Am kommenden Mittwoch (25. Februar) wird der Gesundheitskonzern Fresenius die Zahlen für das vergangene vierte Quartal respektive Gesamtjahr 2025 vorlegen. Der Markt rechnet sowohl umsatz- als auch... ► Artikel lesen | |
| FRESENIUS MEDICAL CARE | 39,840 | +1,58 % | EQS-CMS: Fresenius Medical Care AG: Veröffentlichung einer Kapitalmarktinformation | EQS Zulassungsfolgepflichtmitteilung: Fresenius Medical Care AG
/ Bekanntmachung nach Art. 5 Abs. 1 lit. b) der Verordnung (EU) Nr. 596/2014
Fresenius Medical Care AG: Veröffentlichung... ► Artikel lesen | |
| SIEMENS HEALTHINEERS | 41,370 | -2,41 % | RBC stuft Siemens Healthineers auf 'Outperform' | NEW YORK (dpa-AFX Analyser) - Die kanadische Bank RBC hat Siemens Healthineers mit einem Kursziel von 55 Euro auf "Outperform" belassen. Die Nervosität der Anleger vor einem möglichen Verkauf des Diagnostikgeschäfts... ► Artikel lesen | |
| CARL ZEISS MEDITEC | 25,800 | -5,43 % | Carl Zeiss Meditec AG: ZEISS launches Collaborative Care application to strengthen continuity of care; new solution offers on-premises and cloud-based options | Built on the ZEISS Health Data Platform, ZEISS Collaborative Care offers eye care professionals a flexible way to collaborate - whether as a standalone cloud application or as an integrated... ► Artikel lesen | |
| ECKERT & ZIEGLER | 15,110 | -0,40 % | EQS-AFR: Eckert & Ziegler SE: Vorabbekanntmachung über die Veröffentlichung von Finanzberichten gemäß §§ 114, 115, 117 WpHG | EQS Vorabbekanntmachung Finanzberichte: Eckert & Ziegler SE
/ Vorabbekanntmachung über die Veröffentlichung von Rechnungslegungsberichten
Eckert & Ziegler SE: Vorabbekanntmachung... ► Artikel lesen | |
| UNITEDHEALTH | 252,50 | +1,71 % | Former Optum CEO Heather Cianfrocco to depart UnitedHealth Group | ||
| GERRESHEIMER | 16,120 | +2,68 % | Aktien Frankfurt: Neue US-Zollunsicherheit sorgt für Verluste | FRANKFURT (dpa-AFX) - Der Dax ist am Montag nach einem versöhnlichen Wochenausklang etwas unter Druck geraten. Die Verluste hielten sich aber angesichts der Unsicherheit über neue US-Zölle in Grenzen.... ► Artikel lesen | |
| DRAEGERWERK | 89,50 | 0,00 % | Draegerwerk AG & Co. KGaA: Zollentlastung bereits eingepreist, Aufwärtspotenzial wird kleiner, Kursziel erhöht; auf HALTEN gestuft. | Die Zollerlassungen in den USA bieten einen inkrementellen Unterstützungseffekt für die Draegerwerk, jedoch wird der Nutzen voraussichtlich schrittweise erfolgen. Während bestimmte Zölle aufgehoben... ► Artikel lesen | |
| INTUITIVE SURGICAL | 426,60 | +0,15 % | Im Krankenhaus auf Aktiensuche: Paul Hartmann, Technogym und Intuitive Surgical | Ein paar Dinge habe ich nach meinen Krankenhaus-Aufenthalten im November, Dezember und Januar gelernt...
...zum Beispiel: Es ist egal, wie schlecht es dir geht, es geht immer noch jemandem schlechter... ► Artikel lesen | |
| THERMO FISHER | 434,95 | -1,37 % | THERMO FISHER SCIENTIFIC INC. - 10-K, Annual Report | ||
| TELADOC HEALTH | 4,400 | -0,89 % | Teladoc Health Q4 Loss Narrows | WASHINGTON (dpa-AFX) - Teladoc Health, Inc. (TDOC) on Wednesday reported a fourth-quarter net loss of $25.1 million, or $0.14 per share, compared to $48.4 million or $0.28 per share last year.Fourth-quarter... ► Artikel lesen | |
| STRATEC | 20,650 | -1,90 % | EQS-PVR: STRATEC SE: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective of Europe-wide distribution | EQS Voting Rights Announcement: STRATEC SE
STRATEC SE: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective of Europe-wide... ► Artikel lesen | |
| GENEDX | 74,20 | -6,98 % | These Analysts Slash Their Forecasts On GeneDx After Q4 Results | ||
| PROGYNY | 17,600 | -0,48 % | KeyBanc senkt Kursziel für Progyny-Aktie aufgrund konservativer Schätzungen | ||
| PROCEPT BIOROBOTICS | 25,920 | +14,34 % | PROCEPT BioRobotics Reports Fourth Quarter 2025 Financial Results and Updates 2026 Revenue Guidance | SAN JOSE, Calif., Feb. 25, 2026 (GLOBE NEWSWIRE) -- PROCEPT BioRobotics® Corporation (Nasdaq: PRCT) (the "Company"), a surgical robotics company focused on advancing patient care by developing transformative... ► Artikel lesen |
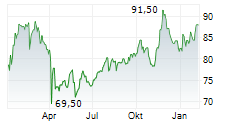
MEDTRONIC PLC gehört der Branche Gesundheitswesen an. Mit einer aktuellen Kursveränderung von +1,29 (+1,56 %) liegt der Kurs bei 83,94 Euro. Beim derzeitigen Kurs ergibt sich, basierend auf einer gesamten Ausschüttung von 2,40 Euro in den vergangenen 12 Monaten, eine Dividendendrendite von +2,86 %.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu MEDTRONIC PLC finden Sie auf Wallstreet Online.