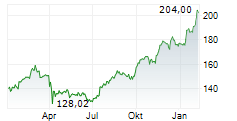
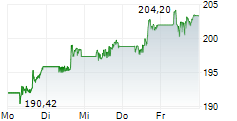
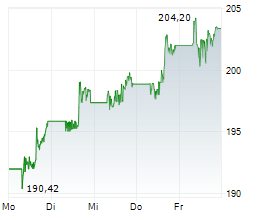
NEW BRUNSWICK (dpa-AFX) - Johnson & Johnson (JNJ) announced the FDA has approved IMAAVY, a human FcRn-blocking monoclonal antibody, for the treatment of generalized myasthenia gravis. The approval is supported by data from the ongoing Vivacity-MG3 study.
David Lee, Global Immunology Therapeutic Area Head, Johnson & Johnson Innovative Medicine, said: 'This approval is the result of years of scientific commitment, collaboration and determination for our nipocalimab program, and we're proud to bring this new treatment option to patients living with anti-AChR or anti-MuSK antibody positive gMG.'
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News