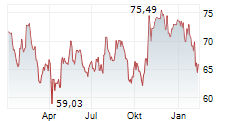
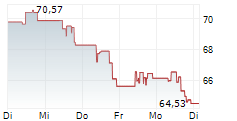
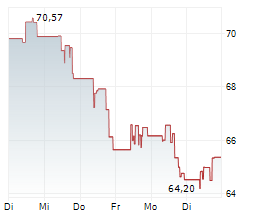
WASHINGTON (dpa-AFX) - Edwards Lifesciences Corp. (EW) Thursday said that the U.S. Food and Drug Administration (FDA) has approved its transcatheter aortic valve replacement (TAVR) therapy, the SAPIEN 3 platform, for severe aortic stenosis (AS) patients without symptoms.
Approval of the SAPIEN 3 platform (SAPIEN 3, SAPIEN 3 Ultra and SAPIEN 3 Ultra RESILIA) is based on positive results from the EARLY TAVR study designed to evaluate TAVR compared to watchful waiting for patients with asymptomatic severe AS. With a median follow-up of 3.8 years, 26.8% of the 455 patients in the TAVR arm experienced death, stroke, or unplanned cardiovascular hospitalization, compared with 45.3% of the 446 patients in the clinical surveillance arm.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News