WASHINGTON (dpa-AFX) - Anavex Life Sciences Corp. (AVXL), a clinical-stage biopharmaceutical company specializing in central nervous system (CNS) disorders, has completed enrollment for its Phase 2 clinical trial evaluating ANAVEX3-71 as a treatment for schizophrenia.
The study, designated ANAVEX3-71-SZ-001, enrolled 71 participants-16 in the completed Part A and 55 in the ongoing Part B, which focuses on extended treatment duration and comprehensive clinical and biomarker data.
ANAVEX3-71 is an oral compound that acts as both a SIGMAR1 receptor agonist and an M1 positive allosteric modulator. Its novel mechanism aims to address all symptom domains of schizophrenia while avoiding the side effects commonly seen with current antipsychotics.
CEO Christopher U Missling, PhD, expressed gratitude to patients and investigators, highlighting the urgent need for more effective schizophrenia treatments and optimism about the drug's potential.
Top-line results from the study are expected in the second half of 2025. The company emphasized that the compound remains investigational, with no assurances of clinical or regulatory success at this stage.
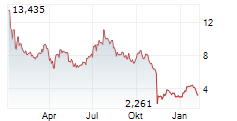
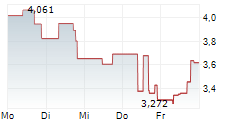
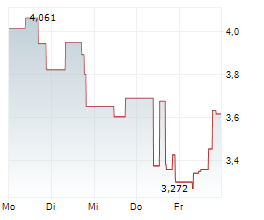
AVXL is currently trading at $9.32, or 1.89% lower on the Nasdaq Global Select Market.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News