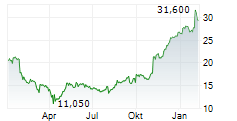
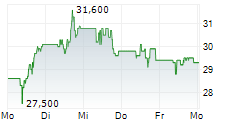
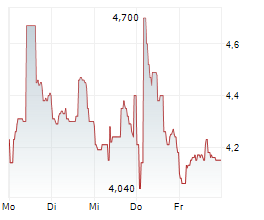
PETAH TIKVA (dpa-AFX) - Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd. (TEVA), and Alvotech (ALVO) Monday said that the U.S. Food and Drug Administration (FDA) has approved Selarsdi as interchangeable with its reference product Stelara .
Selarsdi is approved for all indications matching the reference product, including the treatment of psoriatic arthritis and plaque psoriasis in patients 6 years and older, Crohns disease, and ulcerative colitis.
'Tevas recent launch of two biosimilars SELARSDI and EPYSQLI coupled with a rich pipeline of assets expected to launch over the next few years, position Teva to establish a strong leadership position in the growing landscape of biosimilars and to drive growth for the company as it embarks on the next phase of its strategy,' said Thomas Rainey, Senior Vice President, U.S. Biosimilars at Teva.
'With two important biosimilars on the U.S. market and Biologics License Applications for three new biosimilar candidates under FDA review, Alvotech continues to rapidly expand its portfolio of high-quality biologics based on a fully integrated approach to development and manufacturing, coupled with a unique focus on biosimilars,' said Anil Okay, Chief Commercial Officer for Alvotech.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News