| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
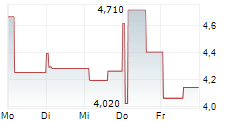
| 3,430 | 3,560 | 24.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 11.02. | Alvotech Announces Increase in Number of Own Shares | 20 | GlobeNewswire (USA) | ||
| 05.02. | Alvotech higher on positive data backing biosimilar to Takeda's Entyvio | 21 | Seeking Alpha | ||
| 05.02. | Alvotech - 6-K, Report of foreign issuer | 5 | SEC Filings | ||
| 05.02. | Alvotech Announces Positive Top-Line Results from Pivotal Pharmacokinetic Study for Proposed Biosimilar to Entyvio | 275 | GlobeNewswire (Europe) | The study, which assessed the pharmacokinetics, safety, tolerability and immunogenicity of AVT80 compared to Entyvio in healthy adult participants, met all its primary endpoints REYKJAVIK, Iceland... ► Artikel lesen | |
| 02.02. | Alvotech Enters Supply, Commercialization Deal With Sandoz Covering Biosimilar Candidates | 16 | RTTNews | ||
| 02.02. | Alvotech enters supply and commercialization agreements for Canada and Australia & New Zealand covering multiple biosimilar candidates | 179 | GlobeNewswire (Europe) | REYKJAVIK, Iceland, Feb. 02, 2026 (GLOBE NEWSWIRE) -- Alvotech (NASDAQ: ALVO), a global biotechnology company specializing in the development and manufacture of biosimilar medicines for patients worldwide... ► Artikel lesen | |
| 29.01. | Alvotech - 6-K, Report of foreign issuer | 7 | SEC Filings | ||
| 29.01. | Alvotech resolves global patent disputes with Regeneron and Bayer over Eylea biosimilar | 54 | Seeking Alpha | ||
| ALVOTECH Aktie jetzt für 0€ handeln | |||||
| 29.01. | Alvotech Secures Settlement Agreement in Global Markets for its Biosimilar to Eylea 2mg | 268 | GlobeNewswire (Europe) | REYKJAVIK, Iceland, Jan. 29, 2026 (GLOBE NEWSWIRE) -- Alvotech (NASDAQ: ALVO), a global biotechnology company specializing in the development and manufacture of biosimilar medicines for patients worldwide... ► Artikel lesen | |
| 06.01. | Alvotech: Transactions of Managers and Closely Associated Persons | 19 | GlobeNewswire (USA) | ||
| 06.01. | Alvotech announces CEO transition | 11 | Seeking Alpha | ||
| 06.01. | Alvotech Appoints Lisa Graver As CEO, Rbert Wessman To Continue As Executive Chairman | 4 | RTTNews | ||
| 06.01. | Alvotech names Lisa Graver as new CEO as founder steps back | 4 | Investing.com | ||
| 06.01. | Alvotech announces planned CEO succession and leadership transition | 413 | GlobeNewswire (Europe) | R- bert Wessman to continue serving as Executive Chairman in full-time capacityLisa Graver appointed Chief Executive Officer REYKJAVIK, ICELAND (January 6, 2026) - Alvotech (NASDAQ: ALVO), a global... ► Artikel lesen | |
| 06.01. | Alvotech Secures $100 Million Term Loan Facility Led By GoldenTree | 13 | pulse2.com | ||
| 02.01. | Alvotech - 6-K, Report of foreign issuer | 7 | SEC Filings | ||
| 31.12.25 | Alvotech Secures Term Loan Facility of USD 100 Million | 6 | GlobeNewswire (USA) | ||
| 29.12.25 | Alvotech: Changes in company's own shares | 14 | GlobeNewswire (USA) | ||
| 23.12.25 | Alvotech - 6-K, Report of foreign issuer | 3 | SEC Filings | ||
| 22.12.25 | Alvotech's Financial Calendar for 2026 | 17 | GlobeNewswire (USA) |
1 2 3 4 5 Weiter >>
111 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,95 | -0,21 % | BioNTech-Konkurrent Moderna gibt kräftig Gas: FDA macht Kehrtwende | Die Aktie des US-Biotech-Unternehmens Moderna kann zur Wochenmitte deutlich zulegen. Moderna hat eine überraschende Kehrtwende der US-Arzneimittelbehörde FDA erreicht: Der Antrag auf Zulassung des mRNA-basierten... ► Artikel lesen | |
| BB BIOTECH | 51,90 | +0,19 % | EQS-News: BB BIOTECH AG: Geschäftsbericht BB Biotech 2025: Starke Performance und Wertrealisierung im Portfolio | EQS-News: BB BIOTECH AG
/ Schlagwort(e): Jahresbericht
Geschäftsbericht BB Biotech 2025: Starke Performance und Wertrealisierung im Portfolio
20.02.2026 / 07:00 CET/CEST
Für... ► Artikel lesen | |
| PAION | 0,080 | -13,61 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 5,020 | -0,40 % | Valneva: Cash-Burn soll 2026 weiter sinken, CEO-Vertrag verlängert | Der französische Impfstoffhersteller Valneva hat für 2025 einen Gesamtumsatz von 174,7 Millionen Euro gemeldet, nach 169,6 Millionen Euro im Vorjahr. Der Produktumsatz ging nach vorläufigen, ungeprüften... ► Artikel lesen | |
| EPIGENOMICS | 1,050 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| BIOFRONTERA | 2,580 | -4,09 % | BIOFRONTERA AG zeigt jetzt strukturelle Stärke | ||
| HEIDELBERG PHARMA | 3,010 | +0,33 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht | ||
| CRISPR THERAPEUTICS | 47,800 | -0,83 % | CRISPR Therapeutics-Aktie: Goldgrube oder Kapitalvernichtung? | Wir sind bekennende GARP-Anhänger (Growth at a Reasonable Price). Nicht aus Prinzip, sondern aus Erfahrung. Wachstum ja - aber bitte nicht um jeden Preis. Wir haben in den letzten zwanzig Jahren genug... ► Artikel lesen | |
| PALATIN TECHNOLOGIES | 22,560 | +1,48 % | Palatin Technologies, Inc.: Palatin Reports Second Quarter Fiscal Year 2026 Financial Results and Provides Corporate Update | Advancing differentiated MC4R-based obesity programs into clinical development
Melanocortin-based obesity therapies targeting rare MC4R pathway disorders with... ► Artikel lesen | |
| BIOXXMED | 1,286 | +16,91 % | XFRA NEW INSTRUMENTS AVAILABLE ON 14.01.2026 | The following instruments on XETRA do have their first trading 14.01.2026 Die folgenden Instrumente in XETRA haben ihren ersten Handelstag 14.01.2026
Aktien
1 MHY3130D1013 Heidmar Maritime Hldgs.... ► Artikel lesen | |
| VIVOSIM LABS | 1,220 | -100,00 % | Morning Market Movers: TechCreate, VivoSim Labs, La Rosa Holdings, Kaixin Holdings See Big Swings | BEIJING (dpa-AFX) - At 7:40 a.m. ET on Friday, premarket trading is seeing notable activity in several stocks, with early price movements signaling potential opportunities before the opening... ► Artikel lesen | |
| INOVIO PHARMACEUTICALS | 1,630 | +1,24 % | Is Inovio Pharmaceuticals Stock Going to $0? | ||
| OCUGEN | 1,527 | +0,76 % | Ocugen Appoints Rita Johnson-Greene to Chief Financial Officer | MALVERN, Pa., Feb. 09, 2026 (GLOBE NEWSWIRE) -- Ocugen, Inc. (Ocugen or the Company) (NASDAQ: OCGN), a pioneering biotechnology leader in gene therapies for blindness diseases, today announced the... ► Artikel lesen | |
| INFLARX | 0,751 | +0,20 % | Weekly Buzz: ARS Pharma's Neffy Goes East, SLS Advances, A Signal Of Hope For IFRX? | LONDON (dpa-AFX) - Despite the typical year-end slowdown, the week saw a steady stream of high-impact news, highlighted by global approvals, clinical trial readouts, new product launches, and... ► Artikel lesen | |
| NOVONESIS | 51,86 | -0,12 % | Novonesis (Novozymes A/S): Novonesis delivered 8% organic sales growth in the first nine months of 2025 | Novonesis delivered 8% organic sales growth in the first nine months of 2025 and narrowed full-year outlook upwards. COPENHAGEN, Denmark - November 6, 2025. Novonesis delivered 8% organic sales growth... ► Artikel lesen |
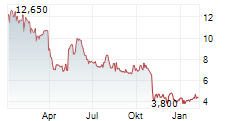
Das Unternehmen ALVOTECH kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell 0,00 % bei einem Aktienkurs von 3,470 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ALVOTECH finden Sie auf Wallstreet Online.