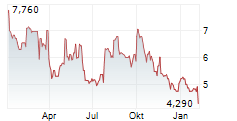
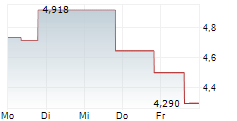
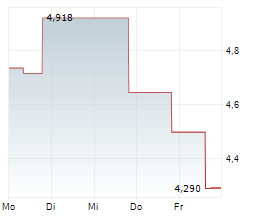
OSE Immunotherapeutics has presented new data for lusvertikimab in ulcerative colitis (UC), with encouraging results from the extension period of the Phase II CoTikiS trial. The key takeaway was that over 90% of patients who achieved a clinical response during the initial 10-week timeframe maintained symptomatic remission through the 24-week extension period. Furthermore, 61% of participants who did not achieve remission during induction achieved it after the extension (on the 850mg dose, the higher of the two tested doses). The safety of lusvertikimab was also confirmed across the 34-week treatment period. In our view, these results demonstrate the potential of lusvertikimab to provide durable responses for UC, thereby strengthening the candidate's data package.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research