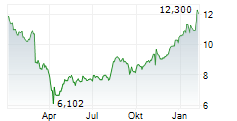
WASHINGTON (dpa-AFX) - Viatris Inc. (VTRS), a healthcare company, Thursday said its two Phase 3 studies evaluating MR-107A-02 for the treatment of acute pain met their primary goals.
The studies were conducted in patients- one following herniorrhaphy surgery and the second following bunionectomy surgery. An opioid comparator arm was also included to confirm the sensitivity of the pain model.
Post-operative herniorrhaphy and bunionectomy patients aged 18 years or older who experienced moderate-to-severe pain following surgery were randomized to receive either MR-107A-02, tramadol, an opioid pain medication, or placebo. In both the pain models, MR-107A-02 showed statistically significant and clinically meaningful improvement in pain compared to placebo. Notable reduction in opioid usage, and superior pain control compared to the opioid arm were also observed.
The company plans to submit a new drug application (NDA) to the the U.S. Food and Drug Administration (FDA) by the end of this year.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News