WASHINGTON (dpa-AFX) - Novo Nordisk A/S (NVO), Monday announced data from the phase 3 REAL8 basket study, a part of the ongoing REAL clinical trial, evaluating the efficacy and safety of once-weekly Sogroya in children with growth disorders.
The study revealed that Sogroya was well-tolerated in participants, with no safety or tolerability issues identified compared to once daily growth hormone.
The trial showed that after 52 weeks, once-weekly Sogroya had similar clinical outcomes and safety profile to once-daily Norditropin in children born small for gestational age, or with Noonan syndrome, or with idiopathic short stature.
Additionally, the trial achieved its primary endpoints for the first three sub-studies, demonstrating that once-weekly Sogroya was non-inferior to once-daily growth hormone treatment at Week 52 across three indications - small for gestational age, Noonan syndrome , and idiopathic short stature.
In April 2025, the company submitted the three indications, based on the data from REAL8 and REAL9, for regulatory review in both the European Union and U.S.
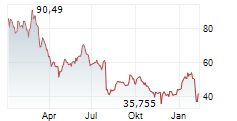
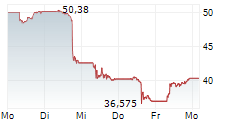
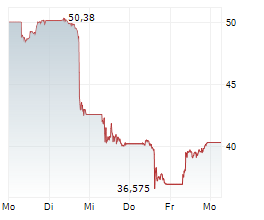
In the pre-market hours, NVO is trading at $63.83, down 2.96 percent on the New York Stock Exchange.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News