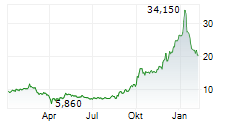
Newron has announced receipt of regulatory clearance to conduct its Phase III programme for evenamide (ENIGMA-TRS), taking it one step closer to the market. It will comprise two separate trials: ENIGMA-TRS 1, a 600-patient international study with enrolment set to start imminently; and ENIGMA-TRS 2, a 400-patient US-focused study with enrolment due to commence within the next three months. Note that the decision to conduct two separate Phase III trials (instead of a widely anticipated single study) was slightly surprising, although we believe this may be strategic with a view to maximise success potential and gain regulatory approval, particularly in the key US market. The 12-week results from the ENIGMA-TRS 1 study, for which we believe the company is well capitalised, are anticipated in Q426, a major upcoming inflection point for Newron. We will present updated estimates in due course based on this latest news.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research