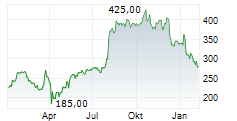
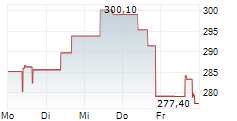
WASHINGTON (dpa-AFX) - Alnylam Pharmaceuticals Inc. (ALNY) announced that results from the HELIOS-B Phase 3 study of AMVUTTRA (vutrisiran) for the treatment of ATTR amyloidosis with cardiomyopathy (ATTR-CM) demonstrate that the drug effectively reduces key cardiovascular (CV) events, including CV hospitalizations and heart failure (HF) hospitalizations.
Additionally, in the analysis, urgent HF visits were reduced by 46% in the overall population during the double-blind period, compared to placebo. The cardiovascular events often precede all-cause mortality (ACM) and are key indicators of disease progression.
Importantly, results from the November 2024 data cut, including further follow up through up to 42 months, reinforce the primary HELIOS-B analysis showing vutrisiran's effect on ACM, and further demonstrate that vutrisiran reduces CV mortality. Through 42 months, the risk of ACM was reduced by 36% and the risk of CV mortality was reduced by 33% in the overall population, compared to placebo.
For both the primary analysis and the current analysis, vital status through 42 months was ascertained for over 99% of all randomized patients from the HELIOS-B study, underscoring the robustness of the results.
The company noted that the analysis of the HELIOS-B Phase 3 study, including mortality data through up to 42 months, was simultaneously published in JACC.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News