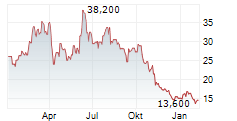
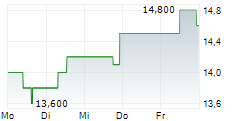
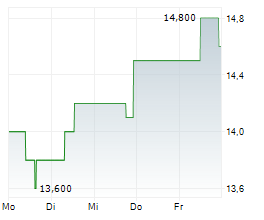
BEIJING (dpa-AFX) - Zai Lab Limited (ZLAB) Monday said that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to ZL-1310 for the treatment of extensive-stage small cell lung cancer (ES-SCLC).
ZL-1310, currently being evaluated in a Phase 1a/1b study, had previously received an Orphan Drug designation for SCLC.
'This designation reinforces the clinical progress we have achieved for ZL-1310 to-date, and we remain on track to initiate a pivotal study in small cell lung cancer later this year, positioning us for a potential accelerated approval in 2027,' said Rafael G. Amado, President, Head of Global Research and Development, Zai Lab.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News