NEW YORK CITY (dpa-AFX) - Drug major Pfizer Inc. (PFE) announced late Monday that it has entered into an exclusive global, ex-China, licensing agreement with Chinese biopharmaceutical company 3SBio, Inc. (TRSBF) for cancer drug SSGJ-707 for up to $6 billion.
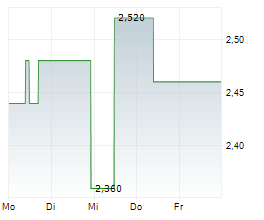
Following the deal, 3SBio shares were gaining around 36 percent in Hong Kong to trade at HK$19.700.
Under the deal terms, 3SBio and its subsidiaries Shenyang Sunshine Pharmaceutical Co., Ltd. and 3S Guojian Pharmaceutical (Shanghai) Co., Ltd. will grant Pfizer an exclusive global license to develop, manufacture and commercialize SSGJ-707 worldwide, excluding China. The agreement also provides Pfizer the option of commercialization rights in China.
3SBio will receive an upfront payment of $1.25 billion and is eligible to receive milestone payments associated with certain development, regulatory and commercial milestones up to $4.8 billion. The transaction also includes tiered double-digit royalties on sales of SSGJ-707, if approved.
The licensing agreement is for the development, manufacturing and commercialization of SSGJ-707, a bispecific antibody targeting PD-1 and VEGF, currently undergoing several clinical trials in China for non-small cell lung cancer, metastatic colorectal cancer, and gynecological tumors.
SSGJ-707 has shown initial efficacy and safety data in a promising class of cancer medicines. 3SBio plans to initiate the first Phase 3 study in China in 2025.
The licensing deal is expected to close in the third quarter subject to fulfillment of customary closing conditions, including receipt of required regulatory approvals and 3SBio shareholder approval.
Upon close, Pfizer will make a $100 million equity investment in 3SBio subject to an agreed upon securities subscription agreement between the parties. Pfizer plans to manufacture drug substance for SSGJ-707 in Sanford, North Carolina, and drug product in McPherson, Kansas.
For More Such Health News, visit rttnews.com
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News