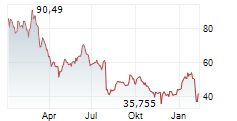
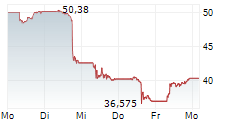
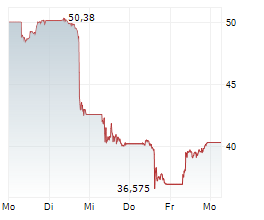
WASHINGTON (dpa-AFX) - Novo Nordisk announced a series of initiatives to further support patient access to FDA-approved Wegovy, or semaglutide, injection 2.4 mg as federal ban on mass compounding of semaglutide takes effect. The company noted that now that the FDA's grace period has ended, any entity that mass produces or sells knockoff semaglutide is breaking the law. Nearly all semaglutide used in compounding is manufactured by Chinese and other foreign suppliers.
Novo Nordisk is offering self-paying patients who are new to Wegovy their first month of medicine for $199 through June 30, 2025. For subsequent months, self-pay patients will pay $499 per month. The offer is designed to help new self-paying patients previously prescribed unapproved semaglutide start on FDA-approved Wegovy.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News