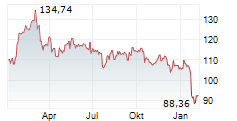
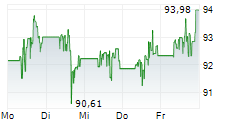
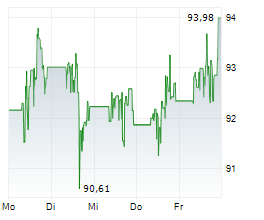
NORTH CHICAGO (dpa-AFX) - Abbott Laboratories (ABT) announced Tuesday that the U.S. Food and Drug Administration (FDA) has approved the company's Tendyne transcatheter mitral valve replacement (TMVR) system to treat people with mitral valve disease.
This life-changing therapy is available for patients whose mitral valves are not functioning properly due to a buildup of calcium in the base of the valves, known as severe mitral annular calcification (MAC).
For patients with severe MAC who are at high risk for open-heart surgery and whose mitral valve cannot be successfully repaired with the Abbott MitraClip device, Tendyne offers an alternative minimally invasive way to replace the valve that's leaky (mitral regurgitation) or narrowed (stenosis).
The innovative and unique design of the Abbott Tendyne system and the valve's availability in multiple sizes allows it to adapt to a range of patient anatomies. The self-expanding valve is delivered through a small incision in the chest and then advanced into the heart to replace the mitral valve.
The valve is fully repositionable and retrievable during implantation, allowing for the best possible outcome for people requiring a valve replacement.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News