WASHINGTON (dpa-AFX) - Beam Therapeutics (BEAM) announced that the FDA has granted orphan drug designation to BEAM-101, an investigational genetically modified cell therapy for the treatment of sickle cell disease.
Amy Simon, chief medical officer of Beam, said: 'We look forward to continuing to progress our BEACON Phase 1/2 clinical trial of BEAM-101 in patients with severe sickle cell disease and to working closely with the FDA with the goal of bringing BEAM-101 to patients as safely and quickly as possible, embodying our mission of providing lifelong cures to patients suffering from severe diseases.'
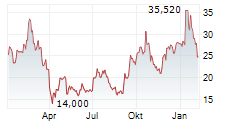
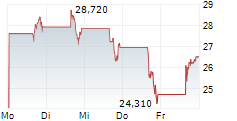
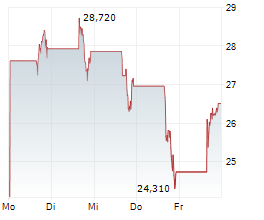
Shares of Beam Therapeutics are up 1% in pre-market trade on Tuesday.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News