TOKYO (dpa-AFX) - Takeda (TAK), Tuesday announced that the European Commission has approved ADCETRIS combined with etoposide, cyclophosphamide, doxorubicin, dacarbazine, and dexamethasone for newly diagnosed Stage IIb, III, and IV Hodgkin lymphoma in adults.
This frontline approval follows a positive CHMP opinion and is based on the Phase 3 HD21 trial, which showed BrECADD's significantly lower treatment-related morbidity and non-inferior progression-free survival compared to the standard eBEACOPP regimen.
Takeda's Global Oncology head, Teresa Bitetti, highlighted the regimen's safety and flexibility, while trial chairman Peter Borchmann, MD, PhD, noted its potential to become a new standard of care by offering improved long-term outcomes with fewer acute toxicities.
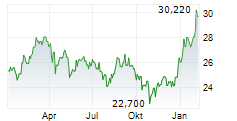
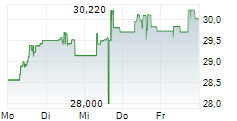
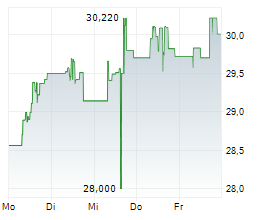
TAK is currently trading at $14.94, down $0.13 or 0.86 percent on the New York Stock Exchange.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News