| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 29,600 | 31,000 | 21:58 | |
| 30,100 | 30,960 | 21:58 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| TAKEDA PHARMACEUTICAL Aktie jetzt für 0€ handeln | |||||
| Mo | Alan Walshe Appointed General Manager of Takeda Canada | 9 | CNW | ||
| Mo | TAKEDA PHARMACEUTICAL COMPANY LIMITED: GENERAL ANNOUNCEMENT::TAKEDA AND PROTAGONIST ANNOUNCE U.S. FDA ACCEPTS NDA AND GRANTS PRIORITY REVIEW FOR RUSFERTIDE | 7 | SGX Company Announcements | ||
| Mo | TAKEDA PHARMACEUTICAL CO LTD - 6-K, Report of foreign issuer | 4 | SEC Filings | ||
| Mo | Takeda, Protagonist Win FDA Priority Review For Rusfertide In Polycythemia Vera | 297 | AFX News | TOKYO (dpa-AFX) - Takeda Pharmaceutical Company Limited (TAK) and Protagonist Therapeutics, Inc. (PTGX) said Monday the U.S. Food and Drug Administration has accepted the New Drug Application... ► Artikel lesen | |
| Do | Protagonist plans to pocket $400M from Takeda rather than split US rusfertide profits | 13 | FiercePharma | ||
| 23.02. | Boston Scientific adds Starbucks CFO, Takeda CEO to board of directors | 18 | MassDevice | ||
| 20.02. | Takeda Shares Down After Entyvio Shows Strong Results In Ulcerative Colitis Trial In Children, Shows Potential To Address Treatment Gaps | 12 | Benzinga.com | ||
| 13.02. | Takeda sichert sich KI-Plattform von Biotech-Startup Iambic für Wirkstoffforschung | 23 | Bionity.com | ||
| 11.02. | Takeda downsizes Boston footprint amid consolidation effort | 18 | FierceBiotech | ||
| 10.02. | FDA kicks off review of Takeda's narcolepsy hopeful | 29 | pharmaphorum | ||
| 10.02. | Takeda signs $1.7bn AI alliance with Iambic | 21 | pharmaphorum | ||
| 10.02. | TAKEDA PHARMACEUTICAL CO LTD - 6-K, Report of foreign issuer | 11 | SEC Filings | ||
| 10.02. | TAKEDA PHARMACEUTICAL COMPANY LIMITED: GENERAL ANNOUNCEMENT::U.S. FDA ACCEPTS NEW DRUG APPLICATION AND GRANTS PRIORITY REVIEW FOR OVEPOREXTON (TAK-861) | 13 | SGX Company Announcements | ||
| 10.02. | Takeda and Iambic announce $1.7bn deal to advance small molecule programmes | 16 | Pharmaceutical Technology | ||
| 10.02. | Takeda Wins FDA Priority Review For Narcolepsy Drug Oveporexton | 335 | AFX News | TOKYO (dpa-AFX) - Takeda Pharmaceutical Company Limited (TAK) said Tuesday that the U.S. Food and Drug Administration has accepted its new drug application for oveporexton and granted Priority... ► Artikel lesen | |
| 09.02. | Takeda, Iambic ink AI drug discovery deal worth more than $1.7B | 14 | Seeking Alpha | ||
| 09.02. | Takeda, Iambic partner in latest pharma AI push | 25 | BioPharma Dive | ||
| 09.02. | Takeda deepens AI drug discovery push with $1.7 billion Iambic deal | 18 | Channel NewsAsia | ||
| 05.02. | Alvotech higher on positive data backing biosimilar to Takeda's Entyvio | 25 | Seeking Alpha | ||
| 02.02. | Takeda Reshapes Leadership, Creates New Units Ahead of Fiscal 2026 | 35 | Contract Pharma |
1 2 3 4 5 Weiter >>
177 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BASF | 45,430 | -4,01 % | DAX vor sehenswertem Monatsplus - BASF spürt schwache Konjunktur | Der DAX wird zum Start in den letzten Handelstag des Monats bei 25 290 Punkten gesehen.
In Asien haben die Investoren insbesondere bei gut gelaufenen Aktien wie den Technologietiteln zum Wochenschluss... ► Artikel lesen | |
| K+S | 15,120 | +0,33 % | K+S: Rohstoffaktie vor dem nächsten Höhenflug? | ||
| SGL CARBON | 3,840 | -5,77 % | SGL Carbon: Neues Kursziel vor den Jahreszahlen | Am 19. März soll es neue Zahlen von SGL Carbon geben. Das Unternehmen rechnet für 2025 mit einem Umsatzminus von 10 Prozent bis 15 Prozent. Im Modell der Analysten stehen -17 Prozent. Das bereinigte... ► Artikel lesen | |
| LINDE | 428,00 | -1,02 % | Vergessen Sie Tech-Aktien! Siemens Energy, A.H.T. Syngas und Linde sind die heimlichen Gelddruckmaschinen | Die Gasspeicher in Deutschland sind auf einem historischen Tiefstand und geopolitische Spannungen den Markt durchwirbeln, zeichnet sich an den Weltmärkten eine paradoxe Lage ab. Ein LNG-Superzyklus... ► Artikel lesen | |
| HENKEL | 77,22 | -4,45 % | Henkel Recalls Tec Italy Shampoo Over Bacterial Contamination | ||
| WACKER CHEMIE | 69,95 | -7,84 % | AKTIEN IM FOKUS: Halbleiterwerte gefragt - Unternehmenszahlen im Blick | FRANKFURT (dpa-AFX) - Aktien von Halbleiterunternehmen haben Anleger am Dienstag auf dem Kaufzettel. Geschäftszahlen und Ausblick von Elmos Semiconductor kamen gut an, die Papiere kletterten auf ein... ► Artikel lesen | |
| LANXESS | 16,830 | -6,66 % | Lufthansa, Rheinmetall, Lanxess, Verbio & Co. - Zwei Szenarien für Ihr Depot im Nahost-Konflikt | Lufthansa, Rheinmetall, Lanxess, Verbio & Co. zwischen Eskalation und Deeskalation. In zwei Szenarien für Ihr Depot im Nahost-Konflikt versuchen wir Chancen aber auch Risiken aufzuzeigen. Wie heute... ► Artikel lesen | |
| COVESTRO | 60,38 | -0,10 % | Covestro AG Q4 Loss Climbs | BRUSSELS (dpa-AFX) - Covestro AG (1COV.DE) announced earnings for its fourth quarter that Increased from the same period last yearThe company's earnings totaled -EUR378 million. This compares... ► Artikel lesen | |
| FUCHS | 36,080 | -1,64 % | ANALYSE-FLASH: Deutsche Bank Research belässt Fuchs SE auf 'Buy' - Ziel 50 Euro | FRANKFURT (dpa-AFX Broker) - Deutsche Bank Research hat die Einstufung für Fuchs SE mit einem Kursziel von 50 Euro auf "Buy" belassen. Mittelfristig dürfte der Schmierstoffhersteller wieder zu seiner... ► Artikel lesen | |
| SYMRISE | 73,20 | -4,31 % | EQS-PVR: Symrise AG: Veröffentlichung gemäß § 40 Abs. 1 WpHG mit dem Ziel der europaweiten Verbreitung | EQS Stimmrechtsmitteilung: Symrise AG
Symrise AG: Veröffentlichung gemäß § 40 Abs. 1 WpHG mit dem Ziel der europaweiten Verbreitung
02.03.2026 / 09:58 CET/CEST
Veröffentlichung... ► Artikel lesen | |
| AIR LIQUIDE | 173,90 | -0,84 % | UBS stuft AIR LIQUIDE(L) auf 'Buy' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat das Kursziel für Air Liquide vor einem Kapitalmarkttag von 200 auf 210 Euro angehoben und die Einstufung auf "Buy" belassen. Die operative... ► Artikel lesen | |
| ALBEMARLE | 142,38 | -6,57 % | Geheime Lithiumaktie: Countdown läuft: Bohrstart im März - steht dieser Junior-Explorer vor der Neubewertung? | ||
| HEXAGON COMPOSITES | 0,707 | -2,08 % | Hexagon Composites: Mutige Trader vor dem Sprung - Kommt jetzt die große Rebound-Chance? | ||
| BRENNTAG | 49,180 | -3,57 % | Westlake Unit Expands India Distribution Partnership With Brenntag | ||
| SQM | 60,40 | -6,79 % | SQM Reports Earnings for the Twelve Months Ended December 31, 2025 | Highlights SQM reported total revenues for the twelve months ended December 31, 2025 of US$4,576.2 million compared to total revenues of US$4,528.8 million for the same period last year. Net income... ► Artikel lesen |
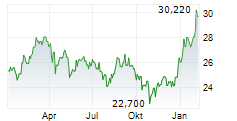
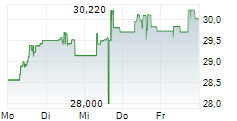
TAKEDA PHARMACEUTICAL CO LTD gehört der Branche Chemie an. Mit einer aktuellen Kursveränderung von -0,860 (-2,74 %) liegt der Kurs bei 30,500 Euro. Beim derzeitigen Kurs ergibt sich, basierend auf einer gesamten Ausschüttung von 1,09 Euro in den vergangenen 12 Monaten, eine Dividendendrendite von +3,57 %.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu TAKEDA PHARMACEUTICAL CO LTD finden Sie auf Wallstreet Online.