WASHINGTON (dpa-AFX) - Dyne Therapeutics (DYN) announced the FDA has granted Breakthrough Therapy Designation to DYNE-101 for the treatment of myotonic dystrophy type 1. The company also announced an updated plan for obtaining U.S. Accelerated Approval for DYNE-101 in DM1 following a Type C meeting with the FDA and analysis of new long-term functional data.
John Cox, president and CEO of Dyne, said: 'Based on feedback from the FDA, along with our 6-month and new 12-month efficacy data, we have submitted a revised protocol for the ongoing Registrational Expansion Cohort of the ACHIEVE trial with vHOT as the primary endpoint for potential Accelerated Approval.'
Dyne plans to use data from the Registrational Expansion Cohort and from the already enrolled patients in the multiple ascending dose and ongoing long-term extension portions of the ACHIEVE trial to support a potential submission for Accelerated Approval in the U.S.
Dyne Therapeutics said data from the cohort are planned for mid-2026 to support a potential U.S. Accelerated Approval submission in late 2026. The company expects that its cash, cash equivalents and marketable securities as of March 31, 2025 will be sufficient to fund its operations into the fourth quarter of 2026.
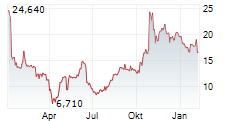
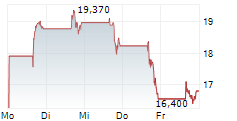
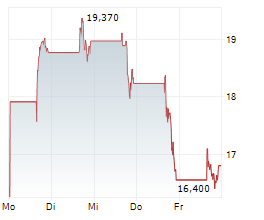
Shares of Dyne Therapeutics are down 24% in pre-market trade on Tuesday.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News