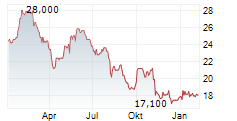
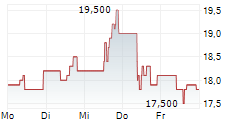
LONDON (dpa-AFX) - Hikma Pharmaceuticals PLC (HIK) Wednesday said that the US Food and Drug Administration (FDA) has approved TYZAVAN, a ready-to-infuse formulation of vancomycin.
TYZAVAN is indicated for treating various bacterial infections in adults and children including septicemia, infective endocarditis, skin and skin structure infections, bone infections, and lower respiratory tract infections.
According to IQVIA, sales of vancomycin injection in the U.S. were nearly $200 million in 2024.
the following infections in adult and pediatric patients (1 month and older) for whom appropriate dosing with this formulation can be achieved: (i) septicemia; (ii) infective endocarditis; (iii) skin and skin structure infections; (iv) bone infections; and (v) lower respiratory tract infections.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News