| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 14,600 | 14,800 | 03.03. | |
| 14,600 | 14,800 | 03.03. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mo | Hikma Pharmaceutical - Total Voting Rights | 5 | RNS | ||
| Do | Hikma Pharmaceuticals reports FY results | 27 | Seeking Alpha | ||
| Do | FTSE 100 movers: LSEG surges on results; Hikma in the red | 7 | Sharecast | ||
| Do | Hikma FY2025 slides: injectables margin reset offsets revenue growth | 30 | Investing.com | ||
| Do | Hikma Pharmaceuticals rejigs top team as shares slide on weak outlook | 7 | Alliance News | ||
| Do | Hikma warns of slower growth, shares plunge | 25 | Sharecast | ||
| HIKMA PHARMACEUTICALS Aktie jetzt für 0€ handeln | |||||
| Do | Hikma Pharma FY25 Earnings Rise; Launches $250 Mln Buyback, Guides FY26; Shares Plunge | 370 | AFX News | LONDON (dpa-AFX) - Hikma Pharmaceuticals PLC (HIK.L), on Thursday reported higher profit for the full year 2025, driven by revenue growth and improved core performance.For the full year 2025... ► Artikel lesen | |
| Do | Hikma names chair chief executive | 7 | Sharecast | ||
| Do | Hikma startet Aktienrückkaufprogramm im Volumen von 250 Millionen US-Dollar | 20 | Investing.com Deutsch | ||
| Do | Hikma launches $250 million share buyback program | 5 | Investing.com | ||
| Do | Hikma Pharmaceutical - Launch of Buyback Programme | 5 | RNS | ||
| Do | Hikma Pharmaceutical - Final Results, Share Buyback & Leadership Changes | 25 | RNS | ||
| 04.02. | Brookfield not planning to make an offer for Hikma Pharmaceuticals | 17 | Sharecast | ||
| 04.02. | Brookfield confirms it will not make offer for Hikma Pharmaceuticals | 5 | Investing.com | ||
| 04.02. | Brookfield bestätigt: Kein Übernahmeangebot für Hikma Pharmaceuticals | 28 | Investing.com Deutsch | ||
| 04.02. | Brookfield Pvt.Cap. - Statement regarding Hikma Pharmaceuticals Plc | 2 | RNS | ||
| 03.02. | Hikma Pharmaceutical - Notice of Results | 18 | RNS | ||
| 20.01. | Supreme Court agrees to review 'skinny label' battle between Hikma, Amarin over generic Vascepa | 32 | FiercePharma | ||
| 07.01. | LONDON BROKER RATINGS: Berenberg raises Vodafone; Barclays cuts Hikma | 37 | Alliance News | ||
| 19.12.25 | Hikma Pharmaceutical - Director/PDMR Shareholding | 11 | RNS |
1 2 3 Weiter >>
57 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 38,310 | -5,66 % | Biotech vor dem Comeback? Was bei Bayer, Vidac und Mesoblast jetzt zählt | ||
| NOVO NORDISK | 31,480 | -0,03 % | maydornsmeinung: Silber, Nvidia, AMD, Microsoft, IBM, SAP, TeamViewer, Novo Nordisk, Nebius & Co. | In maydornsmeinung geht es zunächst um den Gesamtmarkt: Trotz Zoll-Chaos bleiben für Alfred die Unternehmensgewinne der entscheidende Faktor. Die Berichtssaison läuft stark, auch wenn sich die großen... ► Artikel lesen | |
| PFIZER | 22,865 | -0,09 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| NOVARTIS | 141,30 | +0,17 % | Novartis baut fünften Standort für Radioliganden-Therapie in den USA | DJ Novartis baut fünften Standort für Radioliganden-Therapie in den USA
DOW JONES--Der Schweizer Pharmakonzern Novartis hat den Bau eines weiteren Standortes für Radioligandentherapie (RLT) in... ► Artikel lesen | |
| MERCK KGAA | 121,35 | -3,88 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 127,06 | -0,16 % | Gilead Sciences (GILD): Spannendes Chartbild - 150-USD-Trigger im Visier! | Vom HIV-Spezialisten zum Onkologie-Powerhouse! Rückblick Seit Anfang des Jahres befand sich die Aktie von Gilead Sciences in einem starken Aufwärtsimpuls, der sie von rund 122 US-Dollar bis auf ein... ► Artikel lesen | |
| AURORA CANNABIS | 3,015 | +0,17 % | Canaccord Initiates Aurora Cannabis Inc. (ACB) With C$10 Price Target | ||
| SANOFI | 80,70 | +0,29 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,350 | -2,33 % | JPMORGAN stuft GSK auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für GSK nach einer Telefonkonferenz auf "Underweight" mit einem Kursziel von 1700 Pence belassen. Mit einer Übernahme von 35Pharma... ► Artikel lesen | |
| ABBVIE | 202,00 | +0,25 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| ROCHE | 397,55 | +0,20 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| CANOPY GROWTH | 0,917 | 0,00 % | Is Canopy Growth Stock Going to $0? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 868,60 | +0,17 % | Eli Lilly vs. Emyria: Steht die Psychiatrie nach Jahrzehnten der SSRIs vor dem MDMA-Durchbruch? | ||
| MERCK & CO | 103,40 | +0,19 % | Merck skizziert strategische Neuausrichtung nach KEYTRUDA-Patentablauf |
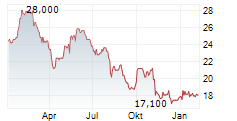
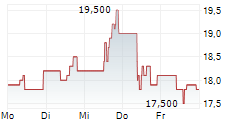
HIKMA PHARMACEUTICALS PLC gehört der Branche Pharma an. Mit einer aktuellen Kursveränderung von +0,100 (+0,68 %) liegt der Kurs bei 14,800 Euro. Beim derzeitigen Kurs ergibt sich, basierend auf einer gesamten Ausschüttung von 0,71 Euro in den vergangenen 12 Monaten, eine Dividendendrendite von +4,80 %.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu HIKMA PHARMACEUTICALS PLC finden Sie auf Wallstreet Online.