CAMBRIDGE (MASSACHUSETTS) (dpa-AFX) - Vertex Pharmaceuticals (VRTX), Thursday reported compelling long-term data for its CRISPR/Cas9 gene-edited therapy, PrCASGEVY, at the EHA Congress.
In sickle cell disease trials, 95.6 percent of 45 evaluable patients remained free of vaso-occlusive crises for at least 12 consecutive months, with an average VOC-free duration of 35 months.
All patients avoided hospitalization for severe crises for an average of 36 months. In transfusion-dependent beta thalassemia, 98.2 percent of 55 patients achieved at least 12 months of transfusion independence, averaging 40.5 months.
Over two-thirds discontinued iron-removal therapy, reflecting improved iron overload. Follow-up now exceeds 5.5 years in SCD and six years in TDT cohorts. The safety profile remains consistent with myeloablative conditioning. PrCASGEVY is approved in multiple countries, with reimbursement secured in several markets.
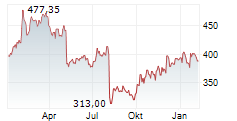
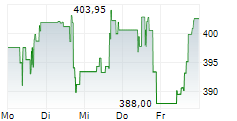
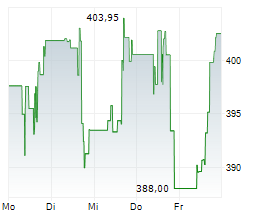
VRTX is currently trading at $458.86, up $1.83 or 0.40 percent on the Nasdaq
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News