COPENHAGEN (dpa-AFX) - Novo Nordisk A/S (NVO), Tuesday announced that it has applied to the European Medicines Agency for a new 7.2?mg dose of its obesity therapy Wegovy, semaglutide, advancing its mission to tailor treatments for people with obesity.
This submission draws on 72-week data from the STEP?UP and STEP?UP?T2D trials, which showed that adults with obesity lost an average of 21 percent of body weight with one-third shedding at least 25 percent versus placebo. The safety profile matched that of the existing 2.4?mg dose.
'The 7.2?mg dose offers additional support for those requiring greater weight loss,' said Ludovic Helfgott, EVP, Product & Portfolio Strategy.
If approved, Novo Nordisk plans a broad EU rollout, aiming to help more patients improve weight and related health outcomes, including cardiovascular, kidney, liver, and joint health.
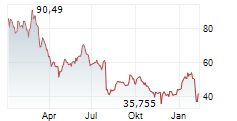
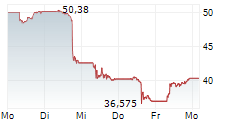
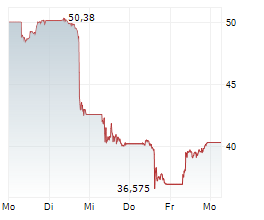
NVO is currently trading at $69.95, up $0.63 or 0.91 percent on the New York Stock Exchange.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News