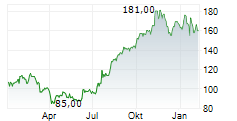
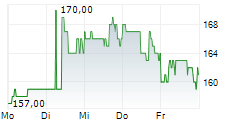
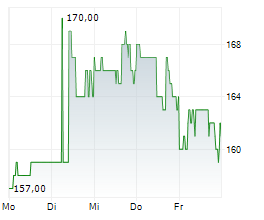
WASHINGTON (dpa-AFX) - Ligand Pharmaceuticals (LGND) announced that its partner Pelthos Therapeutics Inc. (PTHS) has commercially launched ZELSUVMI or berdazimer topical gel 10.3%, the first and only FDA-approved at-home treatment for molluscum contagiosum. Ligand Pharma has earned a $5 million milestone payment from Pelthos following the commercial launch of ZELSUVMI.
Following the completion of the merger between Pelthos Therapeutics and Channel Therapeutics in July 2025, Ligand now owns 56% of Pelthos. Also, under the terms of the license agreement with Pelthos, Ligand is entitled to a 13% royalty on worldwide sales of ZELSUVMI and up to an additional $5 million in commercial sales milestones.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News