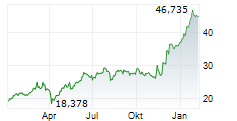
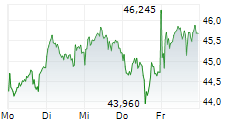
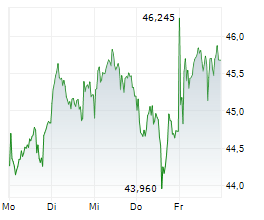
LEVERKUSEN (dpa-AFX) - Bayer announced that the FDA has approved finerenone or Kerendia for the treatment of adult patients with heart failure and a left ventricular ejection fraction of greater than or equal to 40%.
Finerenone is now indicated to reduce the risk of cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in adult patients with heart failure with LVEF of greater than or equal to 40%.
The company said the new indication approval follows the FDA's Priority Review designation and is based on positive results from the Phase III FINEARTS-HF study, which is part of the ongoing MOONRAKER program.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News