DUBLIN (dpa-AFX) - Medtronic (MDT) has announced the enrollment of the first patient in its PELE (PEripheral Onyx Liquid Embolic) clinical trial, which will evaluate the safety and efficacy of the OnyxT Liquid Embolic System or LES for treating arterial hemorrhage in the peripheral vasculature, defined as areas outside the brain and heart.
The initial procedure was performed by Dr. Christopher Stark at Albany Medical Center. Dr. Stark noted that the patient suffered bleeding from a ruptured vessel following a routine procedure. Onyx LES was successfully used to achieve embolization, demonstrating its potential as a critical tool in managing arterial hemorrhage. Albany Medical Center is one of several U.S. sites participating in this study.
The prospective, single-arm, multi-center PELE study will enroll up to 119 patients across 25 U.S. sites. It aims to evaluate primary safety and efficacy outcomes 30 days post-treatment.
David Moeller, president of Medtronic's Peripheral Vascular Health business, said the trial will generate essential clinical evidence for potential FDA approval. While Onyx LES is already approved for peripheral use in many international markets, it remains investigational in the U.S.
The study builds on Medtronic's global experience with Onyx LES and supports the company's broader embolic portfolio strategy.
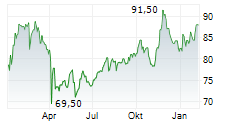
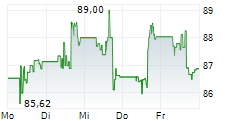
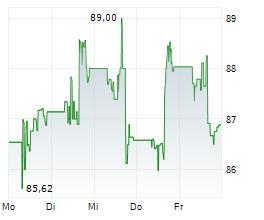
MDT currently trades at $89.54 or 0.36% higher on the NYSE.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News