WASHINGTON (dpa-AFX) - Viatris Inc. (VTRS) announced that a randomized, double-masked, vehicle-controlled, Phase 3 study to evaluate the efficacy and safety of pimecrolimus 0.3% ophthalmic ointment in subjects with blepharitis, did not meet its primary endpoint of complete resolution of debris after six weeks of twice daily dosing.
Viatris Chief R&D Officer Philippe Martin said, 'Given that the study did not meet its objective for patients suffering from blepharitis, we are evaluating the appropriate next steps for the Phase 3 program, which may include revising the planned additional Phase 3 study.'
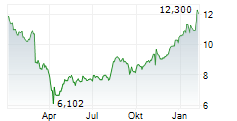
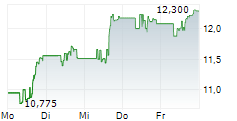
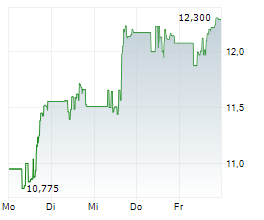
Shares of Viatris are down 3% in pre-market trade on Friday.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News