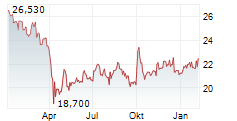
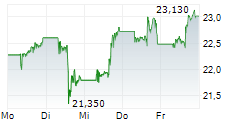
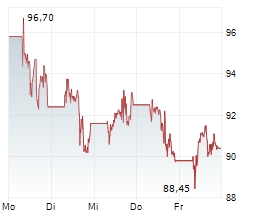
NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and BioNTech SE (BNTX) Friday said that the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for the companies' LP.8.1-adapted COVID-19 vaccine, COMIRNATY LP.8.1, for use in individuals 6 months of age and older.
The adaptation is based on the recommendation of the EMA's Emergency Task Force (ETF) to update COVID-19 vaccines to target the LP.8.1 variant for the 2025-2026 season.
Data showed that the LP.8.1-adapted COVID-19 vaccine generated improved immune response against currently dominant and emerging SARS-CoV-2 lineages compared to 2024-2025 COVID-19 vaccine formulations.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News