COPENHAGEN (dpa-AFX) - Genmab A/S (GMAB), Thursday announced positive results from its Phase 3 EPCORE FL-1 trial evaluating subcutaneous epcoritamab in combination with rituximab and lenalidomide for relapsed or refractory follicular lymphoma.
The study met both primary endpoints, with statistically significant improvements in overall response rate and progression-free survival. The combination reduced the risk of disease progression or death by 79 percent compared to R2 alone.
Data from this interim analysis will be presented at the upcoming ASH annual meeting and support global regulatory submissions. The U.S. FDA has accepted the supplemental Biologics License Application for priority review, with a decision expected by November 30, 2025. If approved, it would mark the first bispecific antibody combo approved in the U.S. for second-line treatment of R/R FL.
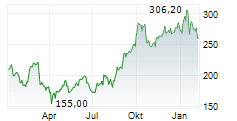
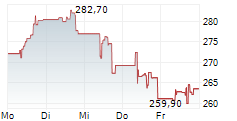
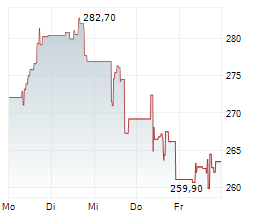
GMAB is currently trading at $22.70, up $1.18 or 5.51 percent on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News