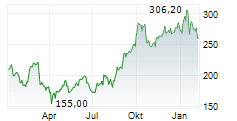
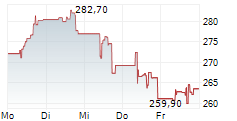
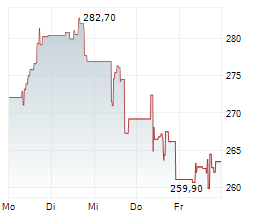
COPENHAGEN (dpa-AFX) - Genmab A/S (GMAB) Tuesday said that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation (BTD) to rinatabart sesutecan for the treatment of adults with endometrial cancer.
The regulatory decision was supported by results from the Phase 1/2 RAINFOL-01 study that showed encouraging responses in heavily pretreated endometrial cancer patients.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News