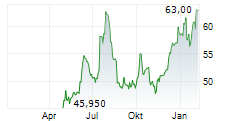
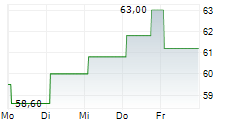
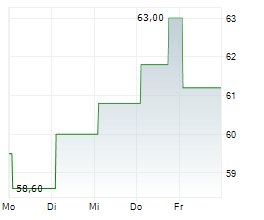
Basilea Pharmaceutica has strengthened its antibacterial pipeline with the acquisition of global rights to the novel, orally administered, Phase-III ready asset, ceftibuten-ledaborbactam etzadroxil. The drug, licensed from Venatorx Pharmaceuticals, is a combination of an oral beta-lactam (cephalosporin) and a beta-lactamase inhibitor (a prodrug of ledaborbactam), aiming to target complicated urinary tract infections (cUTI), including pyelonephritis, caused by multidrug-resistant Enterobacterales. Basilea intends to launch a registrational Phase III programme in c 18 months and expects c CHF15m in incremental R&D spend in FY25 (including an upfront payment and pre-commercial milestones). The deal also includes tiered mid-single-digit royalties and up to US$325m in additional commercial milestones to Venatorx. We are encouraged by the deal and believe an oral and potentially outpatient asset (following intial hospital treatment) broadens the scope of Basilea's otherwise hospital-focused antibacterial franchise. We will provide updated estimates following the H125 results on 19 August.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research