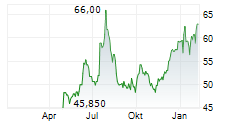
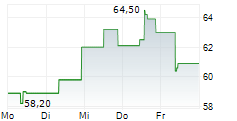
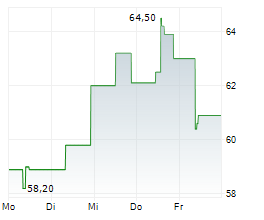
Basilea Pharmaceutica has secured a BARDA contract of up to $159m to advance ceftibuten-ledaborbactam etzadroxil, its recently in-licensed oral, Phase III-ready antibiotic for complicated urinary tract infections (cUTIs). The agreement replaces the original BARDA deal with the licensee Venatorx (October 2023) and provides $6m in committed near-term funding, with a further up to $153m in milestone-based payments, including support for the planned Phase III programme. We believe that this development meaningfully reduces clinical and funding risk for ceftibuten-ledaborbactam, which holds fast track and qualified infectious disease product designations from the FDA. Basilea has also recently received a commitment for a third $25m tranche under its separate $268m OTA funding agreement with BARDA ($93m committed to date), further validating its anti-infective pipeline.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research