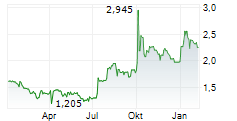
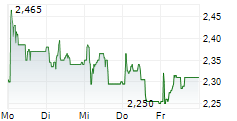
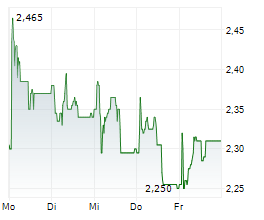
Herantis Pharma has announced that patient visits have been completed for the final cohort from its Phase Ib trial evaluating lead candidate HER-096 in Parkinson's disease (PD). The primary objective of the study is to confirm that repeated subcutaneous doses of HER-096 are safe and well tolerated. Since it is the first test of the candidate in PD patients, the study also entails biomarker analyses, aiming to assess biological responses to the treatment. Top-line results for the trial are anticipated from late September/early October, potentially representing a notable upcoming catalyst for investor attention. The full dataset, including biomarker data, is expected by end-2025, which will likely provide clarity on the next stages of clinical development. We note that, while management is already preparing for Phase II, it is also engaged in potential partnering discussions, seeking the most suitable partnership options to advance the clinical development of HER-096.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research