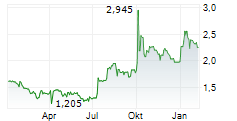
Herantis Pharma has reported encouraging results from the biomarker component of its Phase Ib trial testing HER-096 in patients with Parkinson's disease (PD). The key takeaway was that the data showed that exposure of the drug candidate was associated with key biological changes across various PD-related pathways, including proteostasis, mitochondrial function and neuroinflammation. Importantly, this supports HER-096's mechanism of action as a potential disease-modifier for PD. While these biomarker results should not be confused for efficacy signals, they do provide proof of biological response to HER-096 treatment, confirming the translation of preclinical research through to clinical outcomes, and de-risking the path towards the next stages of clinical development, in our view. Next steps will be a Phase II trial, for which management is actively seeking partnership opportunities. Phase II is expected to commence in 2026.Den vollständigen Artikel lesen ...
© 2026 Edison Investment Research