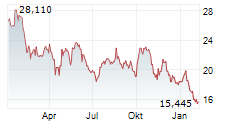
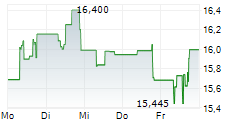
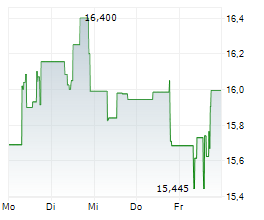
KENILWORTH (NJ) (dpa-AFX) - Daiichi Sankyo announced that Ifinatamab deruxtecan has been granted Breakthrough Therapy Designation by the FDA for the treatment of adult patients with extensive-stage small cell lung cancer with disease progression on or after platinum-based chemotherapy. The FDA granted the BTD based on data from the IDeate-Lung01 phase 2 trial, with support from the IDeate-PanTumor01 phase 1/2 trial.
Ifinatamab deruxtecan is a specifically engineered, potential first-in-class B7-H3 directed DXd antibody drug conjugate discovered by Daiichi Sankyo and being jointly developed by Daiichi Sankyo and Merck (MRK).
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News