| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
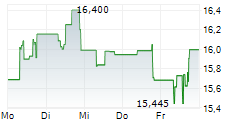
| 15,505 | 15,715 | 08:06 | |
| 15,495 | 15,720 | 08:07 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mo | AstraZeneca and Daiichi secure US fast-track status for cancer drug | 6 | Alliance News | ||
| Mo | Daiichi Sankyo: FDA Grants Priority Review For ENHERTU | 334 | AFX News | LONDON (dpa-AFX) - Daiichi Sankyo (4568.T) and AstraZeneca's (AZN, AZN.L, ZEG.DE, AZN.ST) supplemental Biologics License Application for ENHERTU has been accepted and granted Priority Review... ► Artikel lesen | |
| Fr | Daiichi teams up with GAIA for cholesterol DTx in Europe | 2 | pharmaphorum | ||
| Do | Digitale Unterstützung im Lipidmanagement / Daiichi Sankyo Europe und GAIA kooperieren zur Einführung von lipodia | 182 | news aktuell | München (ots) - - Exklusive Partnerschaft zur Einführung des digitalen Therapeutikums lipodia für Erwachsene mit Hypercholesterinämie - Ziel: Patient:innen über die medikamentöse Therapie hinaus bei... ► Artikel lesen | |
| Do | Daiichi Sankyo Europe GmbH and GAIA: GAIA and Daiichi Sankyo Europe Enter Exclusive Partnership to Launch Next-Generation Digital Therapeutic for Cardiovascular Care in Europe. | 325 | Business Wire | GAIA, the pioneer in evidence-based digital therapeutics and Daiichi Sankyo Europe, today announced an exclusive strategic partnership to commercialize lipodia upon regulatory approval1This press... ► Artikel lesen | |
| 27.02. | Fierce Pharma Asia-China deal growth; Daiichi's new CMO; Astellas-Vir bispecific tie-up | 19 | FiercePharma | ||
| 20.02. | Madrigal sees a stock sell-off; ex-Novartis exec heads to Daiichi | 23 | BioPharma Dive | ||
| 20.02. | Ex-Novartis CMO John Tsai joins Daiichi Sankyo | 5 | pharmaphorum | ||
| DAIICHI SANKYO Aktie jetzt für 0€ handeln | |||||
| 20.02. | Daiichi picks former Novartis CMO John Tsai to head up R&D | 3 | FierceBiotech | ||
| 19.02. | IHH unit moves to block Daiichi Sankyo from obstructing acquisition of Fortis and Malar shares | 13 | The Edge Markets | ||
| 19.02. | Daiichi Sankyo: ENHERTU Type II Variation Application Validated in the EU as Post-Neoadjuvant Treatment for Patients with HER2 Positive Early Breast Cancer | 460 | Business Wire | Based on DESTINY-Breast05 phase 3 trial results, which showed ENHERTU reduced the risk of invasive disease recurrence or death by 53% compared to T-DM1
If approved, Daiichi Sankyo and AstraZeneca's... ► Artikel lesen | |
| 17.02. | BostonGene and Daiichi Sankyo to Bring AI Intel to ADC Development Program | 31 | Contract Pharma | ||
| 17.02. | Daiichi Sankyo setzt auf KI von BostonGene zur Entwicklung von Antikörper-Wirkstoff-Konjugaten | 4 | Investing.com Deutsch | ||
| 17.02. | BostonGene partners with Daiichi Sankyo to advance ADC development | 3 | Investing.com | ||
| 10.02. | DAIICHI Kensetsu Corp. Reports Rise In Nine Months Profit | 21 | RTTNews | ||
| 06.02. | AstraZeneca and Daiichi Sankyo's Datroway given FDA Priority Review for breast cancer treatment | 21 | PMLiVE | ||
| 03.02. | Astra wins FDA priority review for Daiichi's Datroway; denied approval for lupus therapy | 15 | Seeking Alpha | ||
| 03.02. | Daiichi Sankyo, AstraZeneca's Datroway Granted FDA Priority Review In Advanced TNBC | 553 | AFX News | LONDON (dpa-AFX) - Daiichi Sankyo Company Limited (DSKYF) and AstraZeneca's (AZN) supplemental Biologics License Application (sBLA) for Datroway has been granted Priority Review by the U.S.... ► Artikel lesen | |
| 02.02. | Daiichi stops internal development of next-wave ADC as key Datroway readout delayed again | 23 | FierceBiotech | ||
| 30.01. | Daiichi Sankyo GAAP EPS of ¥117.28, revenue of ¥1533.46B; reaffirms FY outlook | 29 | Seeking Alpha |
1 2 3 4 5 Weiter >>
114 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 39,625 | 0,00 % | EQS-HV: Bayer Aktiengesellschaft: Bekanntmachung der Einberufung zur Hauptversammlung am 24.04.2026 in virtuell/Leverkusen mit dem Ziel der europaweiten Verbreitung gemäß §121 AktG | EQS-News: Bayer Aktiengesellschaft
/ Bekanntmachung der Einberufung zur Hauptversammlung
Bayer Aktiengesellschaft: Bekanntmachung der Einberufung zur Hauptversammlung am 24.04.2026... ► Artikel lesen | |
| NOVO NORDISK | 33,205 | +0,41 % | Novo-Nordisk-Aktie: Wer mutig ist, greift jetzt zu! | © Foto: Haberdoedas auf UnsplashDas Kursgeschehen der Aktie von Novo Nordisk ist aktuell nichts für schwache Nerven, doch mutige Anleger kaufen jetzt die ersten Entspannungssignale im Chart.
Novo Nordisk:... ► Artikel lesen | |
| PFIZER | 23,390 | +0,60 % | UBS stuft PFIZER INC auf 'Neutral' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat die Einstufung für Pfizer auf "Neutral" mit einem Kursziel von 25 US-Dollar belassen. Analyst Michael Yee verwies in einer am Dienstag vorliegenden... ► Artikel lesen | |
| NOVARTIS | 135,56 | +0,36 % | Tagesvorschau 6. März: Showdown am Freitag: US-Arbeitsmarkt, Lufthansa-Zahlen und Novartis im Fokus | Entscheidender Börsentag zum WochenschlussDer Wochenschluss steht ganz im Zeichen der US-Konjunktur: Am Nachmittag wird der amerikanische Arbeitsmarktbericht veröffentlicht - ein Datensatz, der regelmäßig... ► Artikel lesen | |
| MERCK KGAA | 111,00 | 0,00 % | Tagesvorschau 5. März: Marktausblick am Donnerstag: DHL, Merck und Aareal Bank öffnen ihre Bücher | Donnerstag: Immobilien, Industrie und WelthandelDer Donnerstag steht ganz im Zeichen großer deutscher Konzerne und wichtiger US-Konjunkturdaten. Mit Zahlen unter anderem von DHL Group, Merck KGaA, Aareal... ► Artikel lesen | |
| GILEAD SCIENCES | 127,86 | -0,27 % | Gilead skizziert strategische Expansionspläne auf der Leerink-Konferenz | ||
| AURORA CANNABIS | 3,060 | +0,16 % | Im Schatten des Ölpreisschocks: Bereiten Tilray, Aurora Cannabis, Canopy & Co. ein spektakuläres Comeback vor? | © Foto: adobe.stock.comDer Iran-Krieg überschattet derzeit das Handelsgeschehen an den Finanzmärkten. Der rasante Anstieg der Ölpreise überschattet so ziemlich alles - auch ein mögliches Comeback der... ► Artikel lesen | |
| SANOFI | 76,86 | +0,97 % | EMA Panel Backs Moderna's COVID-Flu Shot, Sanofi's Sleeping Sickness Drug, Ipsen's Cancer Drug | PARIS (dpa-AFX) - At its February 2026 meeting, the Committee for Medicinal Products for Human Use of the European Medicines Agency adopted positive opinions for Moderna's combined COVID-19... ► Artikel lesen | |
| GSK | 23,820 | 0,00 % | GSK verkauft Rechte an Arzneikandidat für bis zu 690 Mio Dollar | DJ GSK verkauft Rechte an Arzneikandidat für bis zu 690 Mio Dollar
Von Adria Calatayud
DOW JONES--GSK verkauft die Rechte an dem Medikamentenkandidaten Linerixibat an das italienische Pharmaunternehmen... ► Artikel lesen | |
| ABBVIE | 195,40 | -0,31 % | Kampf gegen Pfunde: AbbVie nimmt es mit Eli Lilly und Novo Nordisk auf | Die Pharma-Konzerne Eli Lilly und Novo Nordisk dominieren den Markt für Appetitzügler. Das enorme Potenzial hat inzwischen auch andere Pharma-Konzerne auf den Plan gerufen, die dem Duo mittel- bis langfristig... ► Artikel lesen | |
| ROCHE | 369,00 | +0,30 % | Märkte am Morgen: Lufthansa, Lanxess, Roche, GoPro, StubHub | Nach der kurzen Erholung am Mittwoch sind die Sorgen wegen des Iran-Kriegs zurück am deutschen Aktienmarkt. Der DAX schloss gestern 1,61 Prozent tiefer bei 23.815 Punkten. Heute deutet sich vorbörslich... ► Artikel lesen | |
| CANOPY GROWTH | 0,932 | +2,53 % | Canopy Growth: Is There Any Hope Left for This Beaten-Down Stock? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 859,80 | -0,26 % | EQS-News: WHO Foundation: WHO-Stiftung kündigt Zusammenarbeit mit Lilly an, um Gesundheitssysteme für die Adipositasbehandlung zu stärken | EQS-News: WHO Foundation
/ Schlagwort(e): Joint Venture/Sonstiges
WHO-Stiftung kündigt Zusammenarbeit mit Lilly an, um Gesundheitssysteme für die Adipositasbehandlung zu stärken... ► Artikel lesen | |
| MERCK & CO | 100,80 | 0,00 % | Tempus AI, Inc. (TEM) Announces Strategic Deal with Merck to Fast-Track AI-Driven Precision Medicine |
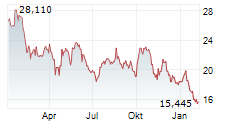
Das Unternehmen DAIICHI SANKYO CO LTD kann der Branche Pharma zugeordnet werden. Die relative Kursveränderung beträgt aktuell -1,18 % bei einem Aktienkurs von 15,500 Euro. Die Dividendendrendite beträgt aktuell +2,77 % (berechnet anhand der Ausschüttung von 0,43 Euro in den letzten 12 Monaten).
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu DAIICHI SANKYO CO LTD finden Sie auf Wallstreet Online.