| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
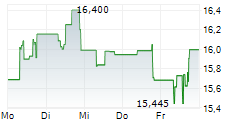
| 14,900 | 15,300 | 17:01 | |
| 15,200 | 15,500 | 15:31 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | Fierce Pharma Asia-China deal growth; Daiichi's new CMO; Astellas-Vir bispecific tie-up | 17 | FiercePharma | ||
| 20.02. | Madrigal sees a stock sell-off; ex-Novartis exec heads to Daiichi | 14 | BioPharma Dive | ||
| 20.02. | Ex-Novartis CMO John Tsai joins Daiichi Sankyo | 5 | pharmaphorum | ||
| 20.02. | Daiichi picks former Novartis CMO John Tsai to head up R&D | 3 | FierceBiotech | ||
| 19.02. | IHH unit moves to block Daiichi Sankyo from obstructing acquisition of Fortis and Malar shares | 13 | The Edge Markets | ||
| 19.02. | Daiichi Sankyo: ENHERTU Type II Variation Application Validated in the EU as Post-Neoadjuvant Treatment for Patients with HER2 Positive Early Breast Cancer | 416 | Business Wire | Based on DESTINY-Breast05 phase 3 trial results, which showed ENHERTU reduced the risk of invasive disease recurrence or death by 53% compared to T-DM1
If approved, Daiichi Sankyo and AstraZeneca's... ► Artikel lesen | |
| 17.02. | BostonGene and Daiichi Sankyo to Bring AI Intel to ADC Development Program | 31 | Contract Pharma | ||
| DAIICHI SANKYO Aktie jetzt für 0€ handeln | |||||
| 17.02. | Daiichi Sankyo setzt auf KI von BostonGene zur Entwicklung von Antikörper-Wirkstoff-Konjugaten | 4 | Investing.com Deutsch | ||
| 17.02. | BostonGene partners with Daiichi Sankyo to advance ADC development | 3 | Investing.com | ||
| 10.02. | DAIICHI Kensetsu Corp. Reports Rise In Nine Months Profit | 21 | RTTNews | ||
| 06.02. | AstraZeneca and Daiichi Sankyo's Datroway given FDA Priority Review for breast cancer treatment | 21 | PMLiVE | ||
| 03.02. | Astra wins FDA priority review for Daiichi's Datroway; denied approval for lupus therapy | 15 | Seeking Alpha | ||
| 03.02. | Daiichi Sankyo, AstraZeneca's Datroway Granted FDA Priority Review In Advanced TNBC | 522 | AFX News | LONDON (dpa-AFX) - Daiichi Sankyo Company Limited (DSKYF) and AstraZeneca's (AZN) supplemental Biologics License Application (sBLA) for Datroway has been granted Priority Review by the U.S.... ► Artikel lesen | |
| 02.02. | Daiichi stops internal development of next-wave ADC as key Datroway readout delayed again | 23 | FierceBiotech | ||
| 30.01. | Daiichi Sankyo GAAP EPS of ¥117.28, revenue of ¥1533.46B; reaffirms FY outlook | 29 | Seeking Alpha | ||
| 30.01. | Daiichi Sankyo Q3 FY2025 slides: Oncology portfolio drives 12.1% revenue growth | 14 | Investing.com | ||
| 30.01. | Onkologie-Portfolio beschert Daiichi Sankyo Umsatzwachstum von 12,1 % | 36 | Investing.com Deutsch | ||
| 30.01. | Daiichi Sankyo Company Limited Announces Increase In Nine Months Income | 440 | AFX News | TOKYO (dpa-AFX) - Daiichi Sankyo Company Limited (DSKYF) reported a profit for its nine months that Increased, from last yearThe company's bottom line totaled JPY217.446 billion, or JPY117.28... ► Artikel lesen | |
| 26.01. | Daiichi Sankyo navigates complexity of success as Enhertu, Datroway push for market dominance | 22 | FiercePharma | ||
| 23.01. | Daiichi Sankyo and AstraZeneca's Enhertu gains NMPA approval for HER2 GEJ | 9 | Pharmaceutical Technology |
1 2 3 4 5 Weiter >>
114 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 38,155 | -6,05 % | Novo Nordisk Aktie: Schon wieder Kurssturz! - Bayer, Hometogo, Indus, Serviceware und TUI - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| NOVO NORDISK | 30,960 | -3,76 % | Lage immer dramatischer: Novo Nordisk - Aktie bricht ein. Das sind die neuen Kursziele | Die Aktie des dänischen Pharmakonzerns Novo Nordisk sorgt weiterhin für Gesprächsstoff. Die schlechten Nachrichten reißen nicht ab. Anleger sind verunsichert und genervt und werfen die Papiere aus ihren... ► Artikel lesen | |
| PFIZER | 22,925 | -1,63 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| NOVARTIS | 139,04 | -2,80 % | HV-Termine: Hauptversammlungen bei BRAIN Biotech, LS Telcom, Metro, MVV Energie, Novartis | Einmal jährlich müssen sich Aufsichtsrat und Vorstand einer Gesellschaft den Aktionären stellen: Die Hauptversammlung ist das höchste Organ einer Aktiengesellschaft und vergleichbarer Unternehmens-Formen.... ► Artikel lesen | |
| MERCK KGAA | 121,20 | -4,00 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 126,72 | -1,26 % | Gilead Sciences (GILD): Spannendes Chartbild - 150-USD-Trigger im Visier! | Vom HIV-Spezialisten zum Onkologie-Powerhouse! Rückblick Seit Anfang des Jahres befand sich die Aktie von Gilead Sciences in einem starken Aufwärtsimpuls, der sie von rund 122 US-Dollar bis auf ein... ► Artikel lesen | |
| AURORA CANNABIS | 3,105 | -2,66 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 80,55 | -1,19 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,670 | -1,04 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| ABBVIE | 201,00 | +0,25 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| ROCHE | 389,95 | -1,79 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| CANOPY GROWTH | 0,896 | -3,55 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 866,80 | -0,42 % | Novo Nordisk setzt Eli Lilly unter Druck | Im frühen US-Handel zählen die Anteilscheine von Eli Lilly zu den schwächsten Titeln. Denn der dänische Rivale Novo Nordisk plant deutliche Preissenkungen für seine Blockbuster-Medikamente in den USA.... ► Artikel lesen | |
| MERCK & CO | 103,40 | -0,39 % | Merck to collaborate with Tempus AI on precision oncology |
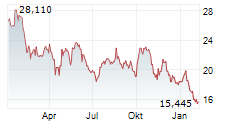
Das Unternehmen DAIICHI SANKYO CO LTD kann der Branche Pharma zugeordnet werden. Mit einer aktuellen Kursveränderung von -0,650 (-4,08 %) liegt der Kurs bei 15,300 Euro. Beim derzeitigen Kurs ergibt sich, basierend auf einer gesamten Ausschüttung von 0,42 Euro in den vergangenen 12 Monaten, eine Dividendendrendite von +2,75 %.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu DAIICHI SANKYO CO LTD finden Sie auf Wallstreet Online.