WASHINGTON (dpa-AFX) - Labcorp Holdings Inc. (LH), Monday announced the availability of the Lumipulse pTau-217/Beta Amyloid 42 Ratio, a blood-based in-vitro diagnostic test to aid in the diagnosis of Alzheimer's disease.
The test, developed by Fujirebio Diagnostics, Inc., is the first of its kind test approved by the U.S. Food and Drug Administration. The company revealed that the test demonstrated a positive predictive value of 92 percent and a negative predictive value of 97 percent during clinical studies.
'By offering this FDA-cleared blood test nationwide, Labcorp is leading the way in delivering innovative solutions for Alzheimer's disease and other neurological conditions by helping patients, families and physicians get answers sooner,' Brian Caveney, chief medical and scientific officer at Labcorp, commented.
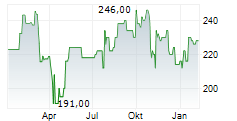
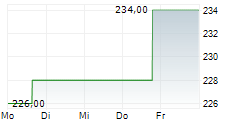
In the pre-market hours, LH is trading at $271.90, up 0.54 percent on the New York Stock Exchange.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News