LUND, Sweden - Immunovia (publ.) the pancreatic cancer diagnostics company, today announced that the PancreaSure test is set to launch in the US on September 2nd. The company's initial focus will be developing advocates for the PancreaSure test among key opinion leaders and top high-risk surveillance centers.
"Setting a launch date for the PancreaSure test and transitioning to commercialization is a key milestone for Immunovia. This step represents the culmination of over two years of work to develop a high-performance, clinically validated early-detection test. Launching with strong support from key opinion leaders and high-risk surveillance centers inspires confidence we are ready to move forward with our commercial strategy," said Jeff Borcherding, CEO of Immunovia.
Immunovia's commercial strategy builds on scalable growth through multiple phases: initial focused investments in KOLs to establish trust and advocacy, followed by expanded selling efforts targeting broader audiences, with parallel initiatives to reach commercial partnerships and secure payer reimbursement in the second half of 2026. Below is an overview of the three-phase strategic rollout of PancreaSure 2025-2027:
- Targeted Advocacy: Initially, Immunovia will focus on key opinion leaders and the 200 high-risk pancreatic cancer surveillance programs in the US. Immunovia will use a targeted, low-cost approach to build advocacy by leveraging the company's existing relationships within the pancreatic cancer community.
- Volume Building: Then, Immunovia will aim to partner with a strategic commercial ally to expand reach and drive adoption among other practitioners and specialists, while building volume among existing users.
- Revenue Growth: Finally, with the support of a commercial partner, Immunovia will increase testing volume within all target groups and secure payer coverage to drive revenue.
At launch, physicians at high-risk pancreatic cancer surveillance centers will implement the PancreaSure test by placing an order in Immunovia's online portal. The high-risk individual's blood will be drawn and then sent to the Immunovia lab in North Carolina. After processing, PancreaSure test results will be shared with the physician in the online portal.
PancreaSure will initially be available as a patient-pay test at the list price of $995. Patients who pay promptly can lower the cost of the test to $750. Immunovia will offer a financial assistance program to lower the cost of the PancreaSure test for those with financial need. Through the financial assistance program, the cost of the test is determined by the patient's ability to pay and can be $0, $100 or $200.
Immunovia plans to begin billing insurance for the PancreaSure test in 2026.
For further information, please contact
Jeff Borcherding, CEO
jeff.borcherding@immunovia.com
Immunovia in brief
Immunovia AB is a diagnostic company whose mission is to increase survival rates for patients with pancreatic cancer through early detection. Immunovia is focused on the development and commercialization of simple blood-based testing to detect proteins and antibodies that indicate a high-risk individual has developed pancreatic cancer. Immunovia collaborates and engages with healthcare providers, leading experts and patient advocacy groups to make its test available to individuals at increased risk for pancreatic cancer.
USA is the world's largest market for detection of pancreatic cancer. The Company estimates that in the USA, 1.8 million individuals are at high-risk for pancreatic cancer and could benefit from annual surveillance testing.
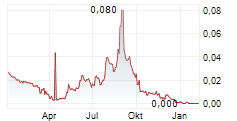
Immunovia's shares (IMMNOV) are listed on Nasdaq Stockholm.
For more information, please visit www.immunovia.com.