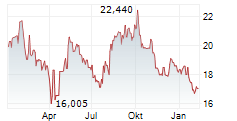
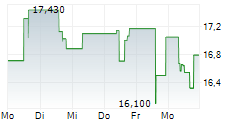
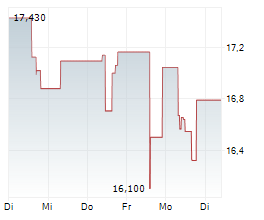
WASHINGTON (dpa-AFX) - Fujifilm Biotechnologies is preparing to open the first phase of its massive biologics manufacturing campus in Holly Springs, North Carolina, a site designed to rank among the largest of its kind in the world.
At the heart of the complex is a central hallway stretching the length of three football fields, connecting four separate buildings that will anchor the company's biologics production.
Two of the facilities are scheduled to begin operations this fall, producing the drug substance, the essential foundation of biologic therapies, for Fujifilm's inaugural customers, Regeneron and Johnson & Johnson. The remaining two buildings are still under construction and slated to open in 2028, expanding the site's production scale and capabilities.
The Holly Springs project reflects Fujifilm's growing ambitions in contract development and manufacturing of biologics, a rapidly expanding market driven by surging demand for advanced therapies, including monoclonal antibodies and cell and gene therapies.
By securing marquee clients such as Regeneron and Johnson & Johnson from the outset, Fujifilm is positioning the facility as a critical hub in the global supply chain for cutting-edge medicines.
North Carolina has become a magnet for biotech investment in recent years, offering a deep talent pool, favorable business environment, and proximity to major pharmaceutical partners. Once fully operational, the Fujifilm site is expected to create thousands of jobs while reinforcing the state's role as a life sciences powerhouse.
With its initial launch just months away, the Holly Springs plant represents both a milestone for Fujifilm's biopharma ambitions and a strategic boost to U.S.-based biologics manufacturing capacity at a time when demand for reliable, large-scale production continues to accelerate.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News