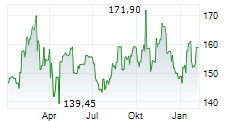
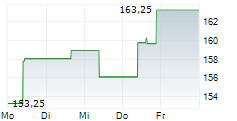
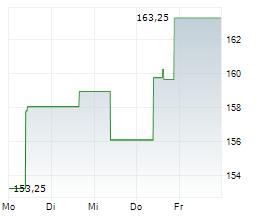
WASHINGTON (dpa-AFX) - Quest Diagnostics (DGX), a provider of diagnostic information services, announced Monday that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the company's Haystack MRD test.
The test helps identify MRD-positive patients with stage II colorectal cancer following curative-intent surgical treatment who may benefit from adjuvant therapy in accordance with therapeutic product labeling.
The new designation adds to growing evidence of the value of the Haystack MRD test for both clinical and pharmaceutical applications. It also aligns with a robust body of research supporting the potential of ctDNA-based MRD tests to detect residual or recurrent cancer from solid tumors.
Quest introduced a clinical laboratory-developed test version of Haystack MRD in late 2024 and is broadening access for oncologists and pharmaceutical partners.
The Breakthrough Devices Program applies to certain medical devices and device-led combination products that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions.
The program is intended to provide patients and healthcare providers with timely access to medical devices by speeding up development, assessment, and review for premarket approval, 510(k) clearance, and De Novo marketing authorization, after undergoing the FDA's rigorous standards for device safety and effectiveness.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News