GOTHENBURG, SE / ACCESS Newswire / August 27, 2025 / IRLAB Therapeutics AB (Nasdaq Stockholm:IRLAB A), a company discovering and developing novel treatments for Parkinson's disease, today announced that the company's interim report for the period January - June 2025, has been published.
KEY HIGHLIGHTS DURING AND AFTER THE SECOND QUARTER
In May, IRLAB was granted another patent that extends the patent protection for the drug candidate mesdopetam in the US.
In May, the company reported positive results from the second part of a Phase I study with IRL757.
In June, a communiqué from the Annual General Meeting was published. All proposals for resolutions were adopted by the AGM. Daniel Johnsson and Catharina Gustafsson Wallich left the Board in connection with the Annual General Meeting and the Board of Directors thereafter consists of Carola Lemne (Chairman), Christer Nordstedt, Gunnar Olsson, Rein Piir and Veronica Wallin.
In June, the Board of Directors resolved, based on the authorization granted by the 2025 Annual General Meeting, on an 85 percent guaranteed rights issue of Class A shares of approximately SEK 136 million.
In June, the company announced that the term of SEK 30 million of the existing loan of SEK 55 million from Fenja Capital was extended until October 30, 2026. The remaining SEK 25 million shall be repaid either by set-off against shares in the Rights Issue or in cash.
In July, the company announced the outcome of the rights issue. With a subscription rate of approximately 61.1% and guarantee undertakings of approximately 23.9 percent of the Rights Issue, the company received approximately SEK 115.7 million before deduction of costs related to the Rights Issue and set-off of loans.
In August, the company announced that Viktor Siewertz leaves the company for a new leading position.
In August, the company announced that Roy Jonebrant will take over as acting CFO on September 1, 2025.
FINANCIAL OVERVIEW OF SECOND QUARTER
Net sales: SEK 19.5m (42.8m)
Operating profit: SEK -25.7m (SEK -5.1m)
Earnings per share before and after dilution: SEK -0.62 (SEK -0.14)
Cash and cash equivalents at the end of the period: SEK 53.6m (SEK 98.3m)
Cash flow from operations: SEK -42.6m (SEK 107m)
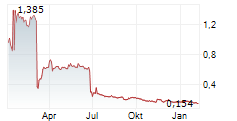
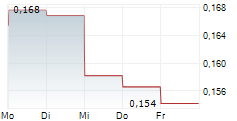
Share price at the end of the period: SEK 3.92 (SEK 13,25)
Figures in brackets = same period 2024, unless otherwise stated.
PRESENTATION TO INVESTORS AND MEDIA
Wednesday, August 27, 2025, at 10.00 CEST a presentation of the interim report will be held through a digital webcast. The presentation will be held in English, followed by a Q&A session.
Access via link: https://www.youtube.com/watch?v=0IkocZF57tA
COMMENTS FROM THE CEO
I have now been CEO of IRLAB for just over a year - a period marked by intensive work, progress, collaborations, and important decisions, which we are now building on with full force. The positive development we saw at the beginning of the year has continued during the second quarter, and I am particularly pleased with the progress we have made in our three most advanced projects. Our portfolio of drug candidates is strong, with several now ready for or approaching out-licensing and partnerships. Thanks to the capital raised from the rights issue carried out this past summer, we can now continue to drive the prioritized activities aimed at establishing partnerships and licensing agreements for our candidates and thereby realizing the commercial value of the projects.
Clinical study in Parkinson's patients with apathy - the next milestone for IRL757
The development of the drug candidate IRL757 is progressing according to plan. We have now compiled the documentation from the two successfully completed and reported Phase I clinical studies, together with preclinical studies supporting the candidate's advancement to the next phase: a study in patients with Parkinson's disease and apathy. Preparing such a clinical study is an extensive undertaking, and after a thorough evaluation process, we have also selected a contract research organization (CRO) to conduct the study.
We look forward with confidence to the next important milestone: the recruitment of the first participant in the trial. Addressing the significant medical need and improving quality of life for people with Parkinson's and apathy is both a challenge and an opportunity to make a real difference.
I want to emphasize the value of our strong partnership with MSRD/Otsuka, where we are responsible for carrying out the development program while they finance the entire development of IRL757 up to and including Phase Ib. We have already received milestone payments and, in connection with the Phase Ib study, will receive an additional SEK 30 million, along with continued funding for its execution.
We confidently look forward to the next important milestone: the recruitment of the first patient, expected to take place during the fourth quarter of 2025.
New patent granted for mesdopetam strengthens commercial value
In May, a new patent for mesdopetam was granted in the US, which strengthens the already robust patent protection for the drug candidate with the potential for market exclusivity well into the 2040s in major and significant markets. This is an important milestone that increases the commercial value of the project.
The strengthened patent protection, the positive feedback from the regulatory authorities in the US and Europe regarding the Phase III program, as well as the favorable positioning and pricing we see for mesdopetam, give us strong reasons to believe we are on the right path. This also adds weight to our ongoing discussions with potential partners.
Our goal is to, through partnerships/out-licensing, advance the development further and, in the future, be able to offer an effective treatment for levodopa-induced dyskinesias (LIDs) - an area with a significant need for new and improved treatment options.
Groundbreaking data and strengthened development plan for pirepemat
Pirepemat, which is being developed to prevent falls and serious fall-related injuries in people with Parkinson's disease, has shown groundbreaking results in the completed Phase IIb REACT-PD study, which aims to identify the optimal dose of the drug candidate for the next phase of development. In the study, we identified the so-called therapeutic window for the plasma levels that individuals need to reach to achieve a significant and highly beneficial effect from the substance. The results were presented this spring at the international AD/PD conference in Vienna, where they attracted considerable interest.
Based on these findings, a strengthened development plan is now in place, and we are preparing to decide on the next step in the development of pirepemat. The work includes preparation for a clinical study with the aim to optimize the titration of individual dosages so that all treated individuals remain within the therapeutic window. The results will be crucial for designing the future Phase III program. The drug candidate has very high market potential since there is currently no available treatment to prevent falls in Parkinson's disease.
Internationally recognized research strengthens our drug candidates
As pioneers in our field, we place great emphasis on participating in international congresses and publishing in scientific journals. During the spring, we have presented clinical results at several congresses, and our employees have co-authored a number of publications. It is gratifying that our research efforts are being recognized, which strengthens both the scientific basis and the value of our unique drug candidates, which are all first-in-class.
Strengthened financing provides additional opportunities for value creation
The strong support in the recently completed rights issue demonstrates the confidence in the potential of our broad project portfolio in the Parkinson's field. With a strengthened financial position and improved capital structure, we are better equipped for discussions with potential partners. At the same time, we have secured resources for the next development stage in two of our unique drug projects, IRL1117 and pirepemat, which will further increase their commercial value and attractiveness. Our main focus is now to secure revenue-generating collaboration agreements based on our medical innovations. I would once again like to extend my sincere thanks to existing and new shareholders, as well as guarantors and lenders, who - despite a challenging external environment - have shown such strong confidence in IRLAB and our strategy.
A big thank you also to Viktor Siewertz, CFO, who, with his legal and financial expertise, strategic insights, and strong relationships with the Swedish and international capital markets, has been an invaluable part of IRLAB's team for more than a decade. Viktor has chosen to leave the company to take on new responsibilities. As of September 1, Roy Jonebrant will take on the role of interim CFO until we have found a permanent solution.
In line with reviewing our costs and priorities to focus our resources on the activities that are most important for our continued development, the Board of Directors decided ahead of the 2025 Annual General Meeting to reduce its fees until license deals or similar transactions have been completed and brought in at least SEK 200 million to the company.
Strong focus on new treatments for Parkinson's disease
It is a privilege to lead a company developing groundbreaking therapies for symptoms in people with Parkinson's disease, where current treatment options are insufficient or entirely lacking. Together with the team, our partners, and owners, I want to continue driving development toward our vision - to transform and improve the lives of people living with Parkinson's and other CNS diseases, while at the same time creating shareholder value by establishing more partnerships and entering into new licensing agreements to realize the commercial potential of our projects.
For more information
Kristina Torfgård, CEO
Phone: +46 730 60 70 99
E-mail: kristina.torfgard@irlab.se
Viktor Siewertz, CFO
Phone: +46 727 10 70 70
E-mail: viktor.siewertz@irlab.se
This information is information that IRLAB Therapeutics is obliged to make public pursuant to the Securities Markets Act. The information was submitted for publication, through the agency of the contact persons set out above, at 2025-08-27 07:00 CEST.
About IRLAB
IRLAB discovers and develops a portfolio of transformative treatments for all stages of Parkinson's disease. The company originates from Nobel Laureate Prof Arvid Carlsson's research group and the discovery of a link between brain neurotransmitter disorders and brain diseases. Mesdopetam (IRL790), under development for treating levodopa-induced dyskinesias, has completed Phase IIb and is in preparation for Phase III. Pirepemat (IRL752), currently in Phase IIb, is being evaluated for its effect on fall frequency in Parkinson's disease. IRL757, a compound being developed for the treatment of apathy in neurodegenerative disorders, is in Phase I. In addition, the company is developing two preclinical programs, IRL942 and IRL1117, towards Phase I studies. IRLAB's pipeline has been generated by the company's proprietary systems biology-based research platform Integrative Screening Process (ISP). Headquartered in Sweden, IRLAB is listed on Nasdaq Stockholm (IRLAB A). For more information, please visit www.irlab.se .
Attachments
IRLAB Q2 FINAL EN
SOURCE: IRLAB Therapeutics
View the original press release on ACCESS Newswire:
https://www.accessnewswire.com/newsroom/en/healthcare-and-pharmaceutical/irlab-publishes-interim-report-for-the-period-january-june-2025-1066113